Biochemistry Principles: Living Organisms & Chemical Foundations
advertisement

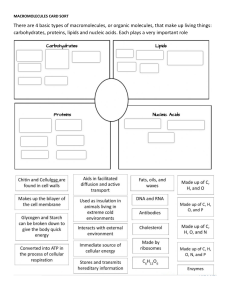
Biochemistry the study of living organisms at the molecular level, has shown us many of the details of the most fundamental processes of life. has shown us how information flows from genes to molecules that have functional capabilities. In recent years, biochemistry has also unraveled some of the mysteries of the molecular generators that provide the energy that powers living organisms. The realization that we can understand such essential life processes has significant philosophical implications. (Tymoczko, John L. et al., 2016) Three principal areas: - - - (1) the structural chemistry of the components of living matter and relationships of biological function to chemical structure; (2) metabolism, the totality of chemical reactions that occur in living matter; and (3) genetic biochemistry, the chemistry of processes and substances that store and transmit biological information. This third area is also the province of molecular genetics, a field that seeks to understand heredity and the expression of genetic information in molecular terms. (Mathews, Christopher K., et al. 2002) What are the Distinguishing Features of Living Organisms? The study of biochemistry shows how the collections of inanimate molecules that constitute living organisms interact to maintain and perpetuate life animated solely by the physical and chemical laws that govern the nonliving universe. Yet organisms possess extraordinary attributes, properties that distinguish them from other collections (Nelson, DL. and Cox, MM., 2008). 1. A high degree of chemical complexity and microscopic organization. Thousands of different molecules make up a cell’s intricate internal structures. These include very long polymers, each with its characteristic sequence of subunits, its unique three-dimensional structure, and its highly specific selection of binding partners in the cell 2. Systems for extracting, transforming, and using energy from the environment. Enabling organisms to build and maintain their intricate structures and to do mechanical, chemical, osmotic, and electrical work. This counteracts the tendency of all matter to decay toward a more disordered state, to come to equilibrium with its surroundings. 3. Defined functions for each of an organism’s components and regulated interactions among them. This is true not only of macroscopic structures, such as leaves and stems or hearts and lungs, but also of microscopic intracellular structures and individual chemical compounds. The interplay among the chemical components of a living organism is dynamic; changes in one component cause coordinating or compensating changes in another, with the whole ensemble displaying a character beyond that of its individual parts. The collection of molecules carries out a program, the end result of which is reproduction of the program and selfperpetuation of that collection of molecules— in short, life. 4. Mechanisms for sensing and responding to alterations in their surroundings, constantly adjusting to these changes by adapting their internal chemistry or their location in the environment. 5. A capacity for precise self-replication and self-assembly. A single bacterial cell placed in a sterile nutrient medium can give rise to a billion identical ―daughter cells in 24 hours. Each cell contains thousands of different molecules, some extremely complex; yet each bacterium is a faithful copy of the original, its construction directed entirely from information contained in the genetic material of the original cell. 6. A capacity to change over time by gradual evolution. Organisms change their inherited life strategies, in very small steps, to survive in new circumstances. The result of eons of evolution is an enormous diversity of life forms, superficially very but fundamentally related through their shared ancestry. This fundamental unity of living organisms is reflected at the molecular level in the similarity of gene sequences and protein structures. phototrophs (Greek trophe , ―nourishment‖ trap and use sunlight, and chemotrophs derive their energy from oxidation of a chemical fuel. Some chemotrophs, the lithotrophs, oxidize inorganic fuels—HS to S0 (elemental sulfur), SO to SO4, NO2 to NO3, or Fe2 to Fe3, for example. Organotrophs oxidize a wide array of organic compounds available in their surroundings. Phototrophs and chemotrophs may also be divided into those that can obtain all needed carbon from CO2 (autotrophs) and those that require organic nutrients (heterotrophs). (Nelson, DL. and Cox, MM., 2008). Three Distinct Domains of Life Two large groups of single-celled microorganisms can be distinguished on genetic and biochemical grounds: Bacteria and Archaea. Bacteria - inhabit soils, surface waters, and the tissues of other living or decaying organisms. Many of the Archaea - recognized as a distinct domain by Carl Woese in the 1980s, inhabit extreme environments—salt lakes, hot springs, highly acidic bogs, and the ocean depths. The available evidence suggests that the Archaea and Bacteria diverged early in evolution. All eukaryotic organisms, which make up the third domain, Eukarya, evolved from the same branch that gave rise to the Archaea; eukaryotes are therefore more closely related to archaea than to bacteria. Within the domains of Archaea and Bacteria are subgroups distinguished by their habitats. In aerobic habitats with a plentiful supply of oxygen, some resident organisms derive energy from the transfer of electrons from fuel molecules to oxygen. Other environments are anaerobic, virtually devoid of oxygen, and microorganisms adapted to these environments obtain energy by transferring electrons to nitrate (forming N2), sulfate (forming H2S), or CO2 (forming CH4). Many organisms that have evolved in anaerobic environments are obligate anaerobes: they die when exposed to oxygen. Others are facultative anaerobes, able to live with or without oxygen. We can classify organisms according to how they obtain the energy and carbon they need for synthesizing cellular material. There are two broad categories based on energy sources: The Unit of Biological Organization: The Cell The two great classes of organisms have different cell types. Prokaryotic cells are uncompartmented; eukaryotes have membrane-bound organelles. The cell can be thought of as a factory, with organelles and compartments specialized to perform different functions. CHEMICAL FOUNDATIONS Biochemistry aims to explain biological form and function in chemical terms. The current understanding that all organisms share a common evolutionary origin is based in part on this observed universality of chemical intermediates and transformations, often termed ―biochemical unity. The four most abundant elements in living organisms, in terms of percentage of total number of atoms, are - hydrogen, oxygen, nitrogen, and carbon, which together make up more than 99% of the mass of most cells. They are the lightest elements capable of efficiently forming one, two, three, and four bonds, respectively; in general, the lightest elements form the strongest bonds. Biomolecules as Compounds of Carbon with a Variety of Functional Groups The chemistry of living organisms is organized around carbon, which accounts for more than half the dry weight of cells. Carbon can form single bonds with hydrogen atoms, and both single and double bonds with oxygen and nitrogen atoms. Of greatest significance in biology is the ability of carbon atoms to form very stable single bonds with up to four other carbon atoms. Two carbon atoms also can share two (or three) electron pairs, thus forming double (or triple) bonds. Covalently linked carbon atoms in biomolecules can form linear chains, branched chains, and cyclic structures. Most biomolecules can be regarded as derivatives of hydrocarbons, with hydrogen atoms replaced by a variety of functional groups that confer specific chemical properties on the molecule, forming various families of organic compounds. Typical of these are alcohols, which have one or more hydroxyl groups; amines, with amino groups; aldehydes and ketones, with carbonyl groups; and carboxylic acids, with carboxyl groups. Many biomolecules are poly functional, containing two or more types of functional groups, each with its own chemical characteristics and reactions. The chemical ―personality‖ of a compound is determined by the chemistry of its functional groups and their disposition in three-dimensional space Receptors convey to the cell that a signal has been received and initiates the cellular response. Thus, for example, insulin binds to its particular receptor, called the insulin receptor, and initiates the biological response to the presence of fuel in the blood. Proteins also play structural roles, allow mobility, and provide defenses against environmental dangers. Perhaps the most prominent role of proteins is that of catalysts—agents that enhance the rate of a chemical reaction without being permanently affected themselves. Protein catalysts are called enzymes. Every process that takes place in living systems depends on enzymes. Carbohydrates Are Fuels and Informational Molecules - Living systems contain a dizzying array of biomolecules. However, these biomolecules can be divided into just four classes: proteins, carbohydrates, nucleic acids and lipids. Proteins Are Highly Versatile Biomolecules - Much of our study of biochemistry will revolve around proteins. Proteins are constructed from 20 building blocks, called amino acids, linked by peptide bonds to form long unbranched polymers. These polymers fold into precise three-dimensional structures that facilitate a vast array of biochemical functions. Proteins serve as signal molecules (e.g., the hormone insulin signals that fuel is in the blood) and as receptors for signal molecules. Most of us already know that carbohydrates are an important fuel source for most living creatures. The most-common carbohydrate fuel is the simple sugar glucose. Glucose is stored in animals as glycogen, which consists of many glucose molecules linked endto-end and has occasional branches. In plants, the storage form of glucose is starch, which is similar to glycogen in molecular composition. There are thousands of different carbohydrates. They can be linked together in chains, and these chains can be highly branched, much more so than in glycogen or starch. Such chains of carbohydrates play important roles in helping cells to recognize one another. Many of the components of the cell exterior are decorated with various carbohydrates that can be identified by other cells and serve as sites of cellto-cell interactions. Nucleic Acids Are the Information Molecules of the Cell - As information keepers of the cell, the primary function of nucleic acids is to store and transfer information. They contain the instructions for all cellular functions and interactions. Like proteins, nucleic acids are linear - molecules. However, nucleic acids are constructed from only four building blocks called nucleotides. A nucleotide is made up of a fivecarbon sugar, either a deoxyribose or a ribose, attached to a heterocyclic ring structure called a base and at least one phosphoryl group. There are two types of nucleic acid: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Genetic information is stored in DNA—the ―parts list‖ that determines the nature of an organism. DNA is constructed from four deoxyribonucleotides, differing from one another only in the ring structure of the bases—adenine (A), cytosine (C), guanine (G), and thymine (T). The information content of DNA is the sequence of nucleotides linked together by phosphodiester linkages. DNA in all higher organisms exists as a double-stranded helix. In the double helix, the bases interact with one another—A with T and C with G. RNA is a single-stranded form of nucleic acid. Some regions of DNA are copied as a special class of RNA molecules called messenger RNA (mRNA). mRNA is a template for the synthesis of proteins. Unlike DNA, mRNA is frequently broken down after use. RNA is similar to DNA in composition with two exceptions: the base thymine (T) is replaced by the base uracil (U), and the sugar component of the ribonucleotides contains an additional hydroxyl (—OH) group. Lipids Are a Storage Form of Fuel and Serve as a Barrier - Among the key biomolecules, lipids are much smaller than proteins or nucleic acids. Whereas proteins and nucleic acids can have molecular weights of thou ands to millions, a typical lipid has a molecular weight of 1300 g mol–1. over, lipids are not polymers made of repeating units, as are proteins and nucleic acids. A key characteristic of many biochemically important lipids is their dual chemical nature: part of the molecule is hydrophilic, meaning that it can dissolve in water, whereas the other part, made up of one or more hydrocarbon chains, is hydrophobic and cannot dissolve in water. This dual nature allows lipids to form barriers that delineate the cell from its environment and to establish intracellular compartments. In other words, lipids allow the development of ―inside‖ and ―outside‖ at a biochemical level. The hydrocarbon chains cannot interact with water and, instead, interact with those of other lipids to form a barrier, or membrane, whereas the water-soluble components interact with the aqueous environment on either side of the membrane. Lipids are also an important storage form of energy. As we will see, the hydrophobic component of lipids can undergo combustion to provide large amounts of cellular energy. Lipids are crucial signal molecules as well The following learning points summarize what you have learned in this section: Biochemistry is the study of living organisms at the molecular level. Biochemistry has three principal areas: (1) the structural chemistry; (2) metabolism; and (3) genetic biochemistry The distinguishing features of living organisms include: a high degree of chemical complexity and microscopic organization; systems for extracting, transforming, and using energy from the environment; defined functions for each of an organism’s components and regulated interactions among them; mechanisms for sensing and responding to alterations in their surrounding; a capacity for precise self-replication and self-assembly; and capacity to change over time by gradual evolution. Prokaryotic cells are uncompartmented; eukaryotic cells have membrane-bound organelles. The three domains of life are: Bacteria; Archaea; and Eukarya. Within the domains of Archaea and Bacteria are subgroups distinguished by their habitats. Organisms can be classified according to how they obtain the energy and carbon they need for synthesizing cellular material. The four most abundant elements in living organisms are hydrogen, oxygen, nitrogen, and carbon, which together make up more than 99% of the mass of most cells. Carbon atoms have the ability to form very stable single bonds with up to four other carbon atoms. Covalently linked carbon atoms in biomolecules can form linear chains, branched chains, and cyclic structures. Biomolecules can be divided into just four classes: proteins, carbohydrates, nucleic acids and lipids.