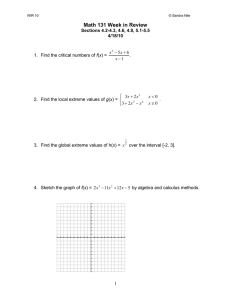
Chapter 2 Solutions Prob. 2.1 (a&b) Sketch a vacuum tube device. Graph photocurrent I versus retarding voltage V for several light intensities. I light intensity Vo V T a his th nd wo o eir is rk w r sa co pro is ill le u vi pr de o rse de ot st f a s d s ec ro n an o te y y p d le d th a a ly by e rt ss fo U in o e r te f t ss th nite gr hi in e ity s w g us d S of or stu e o tat th k ( de f i es e in nt ns co w cl le tr p or ud a uc y r k an ing rnin tors igh d on g. in t la is w D no the iss tea s t p W em ch er or in ing m ld a itt W tio ed id n . e W eb ) Note that Vo remains same for all intensities. (c) Find retarding potential. λ=2440Å=0.244μm =4.09eV 1.24eV μm 1.24eV μm Vo = hν - Φ = -= - 4.09eV = 5.08eV - 4.09eV 1eV λ(μm) 0.244μm Prob. 2.2 Show third Bohr postulate equates to integer number of DeBroglie waves fitting within circumference of a Bohr circular orbit. 4o n 2 mq2 2 4o n 2 rn = mq2 2 rn = and q2 mv2 = and p = mvr 4πo r 2 r n 2 2 4πo rn 2 n 2 2 rn n2 2 = = = mrB2 q2 mrn 2 mv2 m2 v2rn m2 v2rn 2 = n 2 2 mvrn = n p = n is the third Bohr postulate © 2015 Pearson Education, Inc., Hoboken, NJ. All rights reserved. This material is protected under all copyright laws as they currently exist. No portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher. Prob. 2.3 (a) Find generic equation for Lyman, Balmer, and Paschen series. ΔE = hc mq4 = λ 32π2o2 n12 2 - mq4 32π2o 2 n 22 2 mq4 (n 22 - n12 ) mq4 (n 22 - n12 ) hc = = λ 32o 2 n12n 22 2 π2 8o 2 n12n 22 h2 8o 2 n12n 22h2 hc 8εo 2h3c n12n 22 = = mq4 (n 22 - n12 ) mq4 n 22 - n12 8(8.85 10-12 mF )2 (6.63 1034 Js) 3 2.998 108 ms n12n 22 = 2 2 9.11 10-31kg (1.60 10-19C)4 n 2 - n1 n12n 22 n12n 22 = 9.11 Å n 22 - n12 n 22 - n12 n1 =1 for Lyman, 2 for Balmer, and 3 for Paschen T a his th nd wo o eir is rk w r sa co pro is ill le u vi pr de o rse de ot st f a s d s ec ro n an o te y y p d le d th a a ly by e rt ss fo U in o e r te f t ss th nite gr hi in e ity s w g us d S of or stu e o tat th k ( de f i es e in nt ns co w cl le tr p or ud a uc y r k an ing rnin tors igh d on g. in t la is w D no the iss tea s t p W em ch er or in ing m ld a itt W tio ed id n . e W eb ) = 9.11108 m (b) Plot wavelength versus n for Lyman, Balmer, and Paschen series. n 2 3 4 5 n^2 4 9 16 25 LYMAN SERIES n^2-1 n^2/(n^2-1) 3 1.33 8 1.13 15 1.07 24 1.04 LYMAN LIMIT n 3 4 5 6 7 n^2 9 16 25 36 49 BALMER SERIES n^2-4 4n^2/(n^2-4) 5 7.20 12 5.33 21 4.76 32 4.50 45 4.36 BALMER LIMIT 911*n^2/(n^2-1) 1215 1025 972 949 911Ǻ 911*4*n^2/(n^2-4) 6559 4859 4338 4100 3968 n 4 5 6 7 8 9 10 n^2 16 25 36 49 64 81 100 PASCHEN SERIES n^2-9 9*n^2/(n^2-9) 7 20.57 16 14.06 27 12.00 40 11.03 55 10.47 72 10.13 91 9.89 PASCHEN LIMIT 911*9*n^2/(n^2-9) 18741 12811 10932 10044 9541 9224 9010 8199Ǻ 3644Ǻ © 2015 Pearson Education, Inc., Hoboken, NJ. All rights reserved. This material is protected under all copyright laws as they currently exist. No portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher. Prob. 2.4 (a) Find Δpx for Δx=1Ǻ. Δpx Δx = h h 6.6310-34J s Δpx = = = 5.03 10-25 kgsm -10 4 4 Δx 4π 10 m (b) Find Δt for ΔE=1eV. h h 4.14 10-15eV s ΔE Δt = Δt = = = 3.30 10-16s 4 4 ΔE 4π 1eV Prob. 2.5 Find wavelength of 100eV and 12keV electrons. Comment on electron microscopes compared to visible light microscopes. 2 E m h = 2 E m E = 12 mv2 v = h h = = p mv For 100eV, 6.63 10-34J s E- 2 = E- 2 4.9110-19J 2 m 1 1 1 T a his th nd wo o eir is rk w r sa co pro is ill le u vi pr de o rse de ot st f a s d s ec ro n an o te y y p d le d th a a ly by e rt ss fo U in o e r te f t ss th nite gr hi in e ity s w g us d S of or stu e o tat th k ( de f i es e in nt ns co w cl le tr p or ud a uc y r k an ing rnin tors igh d on g. in t la is w D no the iss tea s t p W em ch er or in ing m ld a itt W tio ed id n . e W eb ) λ= 2 9.1110 kg -31 λ = E- 2 4.9110-19J 2 m = (100eV 1.602 10-19 eVJ )- 2 4.9110-19J 2 m = 1.2310-10m = 1.23Å 1 1 For 12keV, 1 1 λ = E- 2 4.9110-19J 2 m = (1.2 104eV 1.602 10-19 eVJ )- 2 4.9110-19J 2 m = 1.12 10-11m = 0.112Å 1 1 1 1 The resolution on a visible microscope is dependent on the wavelength of the light which is around 5000Ǻ; so, the much smaller electron wavelengths provide much better resolution. Prob. 2.6 Which of the following could NOT possibly be wave functions and why? Assume 1-D in each case. (Here i= imaginary number, C is a normalization constant) A) Ψ (x) = C for all x. B) Ψ (x) = C for values of x between 2 and 8 cm, and Ψ (x) = 3.5 C for values of x between 5 and 10 cm. Ψ (x) is zero everywhere else. C) Ψ (x) = i C for x= 5 cm, and linearly goes down to zero at x= 2 and x = 10 cm from this peak value, and is zero for all other x. If any of these are valid wavefunctions, calculate C for those case(s). What potential energy for x ≤ 2 and x ≥ 10 is consistent with this? © 2015 Pearson Education, Inc., Hoboken, NJ. All rights reserved. This material is protected under all copyright laws as they currently exist. No portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher. A) For a wavefunction (x) , we know Ρ = (x)(x)dx = 1 * - Ρ = (x)(x)dx = c * 2 - dx - 0 c = 0 Ρ= (x) cannot be a wave function c 0 B) For 5 x 8 , (x) has two values, C and 3.5C. For c 0 , (x) is not a function and for c = 0 : Ρ = * (x) (x)dx = 0 (x)cannot be a wave function. - iC 3 x-2 2 x 5 C) (x)= iC x-10 5 x 10 5 5 10 2 c2 c 2 2 Ρ = (x)(x)dx = x-2 dx + x-10 dx - 2 9 5 25 T a his th nd wo o eir is rk w r sa co pro is ill le u vi pr de o rse de ot st f a s d s ec ro n an o te y y p d le d th a a ly by e rt ss fo U in o e r te f t ss th nite gr hi in e ity s w g us d S of or stu e o tat th k ( de f i es e in nt ns co w cl le tr p or ud a uc y r k an ing rnin tors igh d on g. in t la is w D no the iss tea s t p W em ch er or in ing m ld a itt W tio ed id n . e W eb ) * 10 c2 c2 3 5 = (x-2) 2 + (x-10)3 5 3×9 3×25 27 125 8c2 = c2 + = 3 27 3×25 8c2 Ρ=1 =1 c=0.612 (x) can be a wave function 3 Since (x) = 0 for x 2 and x 10 , the potential energy should be infinite in these two regions. © 2015 Pearson Education, Inc., Hoboken, NJ. All rights reserved. This material is protected under all copyright laws as they currently exist. No portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher. Prob. 2.7 A particle is described in 1D by a wavefunction: Ψ = Be-2x for x ≥0 and Ce+4x for x<0, and B and C are real constants. Calculate B and C to make Ψ a valid wavefunction. Where is the particle most likely to be? A valid wavefunction must be continuous, and normalized. For (0) = C = B To normalize , 2 dx = 1 - 0 2 8x 2 -4x C e dx + C e dx = 1 - 0 C 1 e + C2 e-4x 1 0 8 4 C2 C2 8 + =1 C= 8 4 3 8x 0 T a his th nd wo o eir is rk w r sa co pro is ill le u vi pr de o rse de ot st f a s d s ec ro n an o te y y p d le d th a a ly by e rt ss fo U in o e r te f t ss th nite gr hi in e ity s w g us d S of or stu e o tat th k ( de f i es e in nt ns co w cl le tr p or ud a uc y r k an ing rnin tors igh d on g. in t la is w D no the iss tea s t p W em ch er or in ing m ld a itt W tio ed id n . e W eb ) 2 Prob. 2.8 The electron wavefunction is Ceikx between x=2 and 22 cm, and zero everywhere else. What is the value of C? What is the probability of finding the electron between x=0 and 4 cm? = Ceikx 22 -1 1 cm 20 dx = C (20) = 1 C = * 2 2 2 1 1 Probability = dx = 2 = 10 20 0 4 2 Prob. 2.9 Find the probability of finding an electron at x<0. Is the probability of finding an electron at x>0 zero or non-zero? Is the classical probability of finding an electron at x>6 zero or non? The energy barrier at x=0 is infinite; so, there is zero probability of finding an electron at x<0 (|ψ|2 =0). However, it is possible for electrons to tunnel through the barrier at 5<x<6; so, the probability of finding an electron at x>6 would be quantum mechanically greater © 2015 Pearson Education, Inc., Hoboken, NJ. All rights reserved. This material is protected under all copyright laws as they currently exist. No portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher. than zero (|ψ|2 >0) and classical mechanically zero. Prob. 2.10 Find 4 px2 2 pz 2 7mE for ( x, y, z, t ) A e j (10x3 y-4t ) . j(10x+3y-4t) dx j x A e - A e = = 100 2 - j(10x+3y-4t) j(10x+3y-4t) e dx A e - - j(10x+3y-4t) - - j(10x+3y-4t) A e * E = 2 2 j(10x+3y-4t) dz j z A e - A e = =0 2 - j(10x+3y-4t) j(10x+3y-4t) e dz A e * pz 2 T a his th nd wo o eir is rk w r sa co pro is ill le u vi pr de o rse de ot st f a s d s ec ro n an o te y y p d le d th a a ly by e rt ss fo U in o e r te f t ss th nite gr hi in e ity s w g us d S of or stu e o tat th k ( de f i es e in nt ns co w cl le tr p or ud a uc y r k an ing rnin tors igh d on g. in t la is w D no the iss tea s t p W em ch er or in ing m ld a itt W tio ed id n . e W eb ) px 2 2 - j(10x+3y-4t) * - j(10x+3y-4t) dt j t A e = 4 2 - j(10x+3y-4t) j(10x+3y-4t) A e e dt - 4 px 2 +2 pz 2 +7mE = 400 2 28(9.1110-31kg) © 2015 Pearson Education, Inc., Hoboken, NJ. All rights reserved. This material is protected under all copyright laws as they currently exist. No portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher. Prob. 2.11 Find the uncertainty in position (Δx) and momentum (Δρ). 2 πx sin e-2πjEt/h and L L Ψ(x,t) = L x = * x dx = 0 L Δx = p x2 - x 2 dx = 1 * 0 2 x x sin 2 dx = 0.5L (from problem note) L0 L x 2 = * x dx = 0 L L 2L 2 x x sin 2 dx = 0.28L2 (from problem note) L0 L = 0.28L2 - (0.5L)2 = 0.17L h h = 0.47 4π Δx L T a his th nd wo o eir is rk w r sa co pro is ill le u vi pr de o rse de ot st f a s d s ec ro n an o te y y p d le d th a a ly by e rt ss fo U in o e r te f t ss th nite gr hi in e ity s w g us d S of or stu e o tat th k ( de f i es e in nt ns co w cl le tr p or ud a uc y r k an ing rnin tors igh d on g. in t la is w D no the iss tea s t p W em ch er or in ing m ld a itt W tio ed id n . e W eb ) Prob. 2.12 Calculate the first three energy levels for a 10Ǻ quantum well with infinite walls. En = n 2 π2 2 (6.63 10-34 )2 = n 2 = 6.03 10-20 n 2 2 m L2 8 9.111031 (109 )2 E1 = 6.03 10-20J = 0.377eV E2 = 4 0.377eV = 1.508eV E3 = 9 0.377eV = 3.393eV Prob. 2.13 Show schematic of atom with 1s22s22p4 and atomic weight 21. Comment on its reactivity. nucleus with 8 protons and 13 neutrons 2 electrons in 1s 2 electrons in 2s This atom is chemically reactive because the outer 2p shell is not full. It will tend to try to add two electrons to that outer shell. 4 electrons in 2p = proton = neturon = electron © 2015 Pearson Education, Inc., Hoboken, NJ. All rights reserved. This material is protected under all copyright laws as they currently exist. No portion of this material may be reproduced, in any form or by any means, without permission in writing from the publisher.