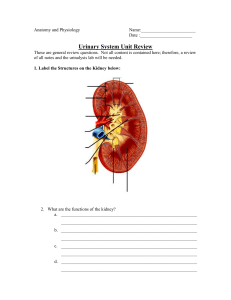
60: Assessment of the Renal/Urinary System Carolyn Gersch LEARNING OUTCOMES 1. Collaborate with the interprofessional team to perform a complete urinary and renal system assessment, including elimination, fluid and electrolyte balance, and acid-base balance. 2. Provide a safe environment for patients and staff when performing a physical assessment of the renal and urinary systems. 3. Explain how physiologic changes of the urinary/renal system affect elimination and the associated care of older adults. 4. Implement patient-centered nursing interventions to help patients and families cope with the psychosocial impact caused by a urinary elimination health problem. 5. Apply knowledge of anatomy and physiology to perform an evidencebased assessment for the patient with a urinary elimination health problem. 6. Use clinical judgement to analyze assessment findings for the patient with a urinary or renal system health problem. 7. Teach the patient and caregivers about diagnostic procedures associated with assessment of kidney and urinary health problems. KEY TERMS bruit An audible swishing sound produced when the volume of blood or the diameter of the blood vessel changes. calculi Stone formation. continence cystitis The ability to voluntarily control emptying of the bladder or colon. A bladder inflammation, most often with infection. elimination The excretion of waste from the body by the GI tract (as feces) and kidneys (as urine). external urethral sphincter Skeletal muscle that surrounds the urethra and helps to control the exit of urine. incontinence The involuntary loss of urine or stool. internal urethral sphincter is smooth detrusor muscle of the bladder neck and elastic tissue that helps to control the exit of urine. microalbuminuria The presence of very small amounts of albumin in the urine that are not measurable by usual urinalysis procedures. nocturnal polyuria renal threshold Increased urination at night. The point at which the kidney is overwhelmed with glucose and can no longer reabsorb; also called transport maximum. proteinuria uremia The presence of protein in the urine. The buildup of nitrogenous waste products in the blood (azotemia). urethral meatus urgency The opening at the endpoint of the urethra. A sense of a nearly uncontrollable need to urinate. Priority and Interrelated Concepts The priority concept for this chapter is: • Elimination The interrelated concepts for this chapter are: • Fluid and Electrolyte Balance • Acid-Base Balance The concept of elimination is the excretion of waste from the body by the GI tract as feces and by the kidneys as urine. See Chapter 3 for an overview of the concept of elimination and how it relates to the concepts of fluid and electrolyte balance and acid-base balance. In this chapter, the focus is on elimination of waste via the renal system. The kidneys and urinary tract make up the renal system, which is responsible for urine elimination. The kidneys are responsible for filtering water and wastes from the bloodstream. The filtered-out particles and excess fluid are excreted out of the body into the urinary tract via the ureters, bladder, and ureters ridding the body of these wastes. Structural or functional problems within the renal system may alter fluid and electrolyte balance and acid-base balance. The kidneys help maintain health in many ways. Most important, they maintain body fluid volume and composition and create urine for waste elimination. In addition, the kidneys help regulate blood pressure, acidbase balance; produce erythropoietin for red blood cell (RBC) synthesis; and convert vitamin D to an active form. Anatomy and Physiology Review Kidneys Structure The two kidneys are located behind the peritoneum, outside of the abdominal cavity, one on either side of the spine (Fig. 60.1). The adult kidney is 4 to 5 inches (10 to 13 cm) long, 2 to 3 inches (5 to 7 cm) wide, and about 1 inch (2.5 to 3 cm) thick. The left kidney is slightly longer and narrower than the right kidney. Larger-than-usual kidneys may indicate obstruction or polycystic disease. Smaller-than-usual kidneys may indicate chronic kidney disease (CKD). Variation in kidney shape and number is relatively common and does not always indicate a problem in kidney function. Some adults have more than two kidneys or may have only one large, horseshoe-shaped kidney. As long as tests of kidney function are normal, these variations are of no significance (Brenner, 2016). Several layers of tissue surround the kidney, providing protection and support. The outer surface of the kidney is a layer of fibrous tissue called the capsule (Fig. 60.2). It covers most of the kidney except the hilum, which is the indented area where the kidney blood vessels and nerves enter and exit. It is also where the ureter exits. The capsule is surrounded by layers of fat and connective tissue. Underneath the capsule fibrous layer are two layers of functional kidney tissue: the cortex and the medulla. The renal cortex is the outer tissue layer. The medulla is the medullary tissue lying below the cortex in the shape of many fans. Each “fan” is called a pyramid. Pyramids are separated by the renal columns, cortical tissue that dips down into the interior of the kidney. The tip of each pyramid is called a papilla. The papillae drain urine into the collecting system. A cuplike structure called a calyx collects the urine at the end of each papilla. The calices join together to form the renal pelvis, which narrows to become the ureter. FIG. 60.1 Anatomic location of the kidneys and structures of the urinary system. FIG. 60.2 Bisection of the kidney showing its major structures. The kidneys have a rich blood supply and receive a blood flow from 600 to 1300 mL/min. The blood supply to each kidney comes from the renal artery, which branches off from the abdominal aorta. The renal artery divides into progressively smaller arteries, supplying blood to areas of the kidney tissue and the nephrons. The smallest arteries (afferent arterioles) feed the nephrons directly to form urine. Venous blood from the kidneys starts with the capillaries surrounding each nephron. These capillaries drain into progressively larger veins, with blood eventually returned to the inferior vena cava through the renal vein. Microscopic Anatomy The nephron is the functional unit of the kidney and forms urine by filtering waste products and water from the blood. There are about 1 million nephrons per kidney, and each nephron separately performs filtration and makes urine from blood. There are two types of nephrons: cortical nephrons and juxtamedullary nephrons. The cortical nephrons are short and lie totally within the renal cortex. The juxtamedullary nephrons (about 20% of all nephrons) are longer, and their tubes and blood vessels dip deeply into the medulla. The purpose of these nephrons is to concentrate urine during times of low fluid intake to allow continued excretion of body waste with less fluid loss (McCance & Huether, 2019). Blood supply to the nephron is delivered through the afferent arteriole (i.e., the smallest, most distal portion of the renal arterial system). From the afferent arteriole, blood flows into the glomerulus, which is a series of specialized capillary loops. It is through these capillaries that water and small particles are filtered from the blood to make urine. The remaining blood leaves the glomerulus through the efferent arteriole, which is the first vessel in the kidney’s venous system. From the efferent arteriole, blood exits into either the peritubular capillaries around the tube of the cortical nephrons or the vasa recta around the tube of juxtamedullary nephrons. Each nephron is a tubelike structure with distinct parts (Fig. 60.3). The tube begins with the Bowman capsule, a saclike structure that surrounds the glomerulus. The tubular tissue of the Bowman capsule narrows into the proximal convoluted tubule (PCT). The PCT twists and turns, finally straightening into the descending limb of the loop of Henle. The descending loop of Henle dips in the direction of the medulla but forms a hairpin loop and comes back up into the cortex as the ascending loop of Henle. The two segments of the ascending limb of the loop of Henle are the thin segment and the thick segment. The distal convoluted tubule (DCT) forms from the thick segment of the ascending limb of the loop of Henle. The DCT ends in one of many collecting ducts located in the kidney tissue. The urine in the collecting ducts passes through the papillae and empties into the renal pelvis. Special cells in the afferent arteriole, efferent arteriole, and DCT are known as the juxtaglomerular complex (Fig. 60.4). These cells produce renin, which is a hormone that helps regulate blood flow, glomerular filtration rate (GFR), and blood pressure. Renin is secreted when sensing cells in the DCT (called the macula densa) sense changes in blood volume and pressure. The macula densa touches the renin-producing cells. Renin is produced when the macula densa cells sense that blood volume, blood pressure, or blood sodium level is low. Renin then converts renin substrate (angiotensinogen) into angiotensin I. This leads to a series of reactions that cause secretion of the hormone aldosterone (Fig. 60.5). Aldosterone increases kidney reabsorption of sodium and water, restoring blood pressure, blood volume, and blood sodium levels (McCance & Huether, 2019). It also promotes excretion of potassium (see Chapter 13). FIG. 60.3 Anatomy of the nephron—the functional unit of the kidney. The differences in appearance in tubular cells seen in a cross section reflect the differing functions of each nephron segment. Note that the particular nephron labeled here is a juxtamedullary nephron. From Patton, K. T., & Thibodeau, G. A. [2018]. The human body in health & disease [7th ed.]. St. Louis: Mosby. FIG. 60.4 Juxtaglomerular complex showing juxtaglomerular cells and the macula densa. FIG. 60.5 Role of aldosterone, renin substrate (angiotensinogen), angiotensin I, and angiotensin II in the renal regulation of water and sodium. The glomerular capillary wall has three layers (Fig. 60.6): the endothelium, the basement membrane, and the epithelium. The endothelial and epithelial cells lining these capillaries are separated by pores that filter water and small particles from the blood into the Bowman capsule. This fluid is called the filtrate. Function The kidneys have both regulatory and hormonal functions. The regulatory functions control fluid and electrolyte balance and acid-base balance. The hormonal functions control red blood cell (RBC) formation, blood pressure, and vitamin D activation. Regulatory Functions The kidney processes that maintain fluid and electrolyte balance and acidbase balance through urine elimination are glomerular filtration, tubular reabsorption, and tubular secretion. These processes use filtration, diffusion, active transport, and osmosis. (See Chapter 13 for a review of these actions.) Table 60.1 lists the functions of nephron tubules and blood vessels. Glomerular filtration is the first process in urine formation. As blood passes from the afferent arteriole into the glomerulus, water, electrolytes, and other small particles (e.g., creatinine, urea nitrogen, glucose) are filtered across the glomerular membrane into the Bowman capsule to form glomerular filtrate. As the filtrate enters the proximal convoluted tubule (PCT), it is called tubular filtrate or early urine. Large particles, such as blood cells, albumin, and other proteins, are too large to filter through the glomerular capillary walls. Therefore these substances are not normally present in the excreted final urine. FIG. 60.6 Glomerular capillary wall. Filtration rate is expressed in milliliters per minute. Normal glomerular filtration rate (GFR) averages 125 mL/min, totaling about 180 L daily. If the entire amount of filtrate were excreted as urine, death would occur from dehydration. Actually, only about 1 to 3 L are excreted each day as urine. The rest is reabsorbed back into the blood (McCance & Huether, 2019). GFR is controlled by blood pressure and blood flow. The kidneys selfregulate their own blood pressure and blood flow, which keeps GFR constant. GFR is controlled by selectively constricting and dilating the afferent and efferent arterioles. When the afferent arteriole is constricted or the efferent arteriole is dilated, pressure in the glomerular capillaries falls and filtration decreases. When the afferent arteriole is dilated or the efferent arteriole is constricted, pressure in the glomerular capillaries rises and filtration increases. This way the kidney maintains a constant GFR, even when systemic blood pressure changes. When systolic pressure drops below 65 to 70 mm Hg, these self-regulation processes do not maintain GFR. TABLE 60.1 Vascular and Tubular Components of the Nephron Tubular reabsorption is the second process in urine formation. Tubular reabsorption of most of the filtrate (early urine) keeps normal urine output at 1 to 3 L/day and prevents dehydration. As the filtrate passes through the tubular parts of the nephron, water and electrolytes are reabsorbed from the tubular lumen of the nephron and into the peritubular capillaries. This process returns much of the water, electrolytes, and other particles to the blood. The tubules return about 99% of filtered water back into the body (Fig. 60.7). Most water reabsorption occurs in the proximal convoluted tubule (PCT). Water reabsorption continues as the filtrate flows down the descending loop of Henle. The thin and thick segments of the ascending loop of Henle are not permeable to water, and no water reabsorption occurs here. The distal convoluted tubule (DCT) can be permeable to water, and some water reabsorption occurs as the filtrate continues to flow through the tubule. The membrane of the DCT may be made more permeable to water when vasopressin (antidiuretic hormone [ADH]) and aldosterone are present. Vasopressin increases tubular permeability to water, allowing water to leave the tube and be reabsorbed into the capillaries. Vasopressin also increases arteriole constriction. Arteriole constriction alters blood pressure, which then affects the amounts of fluid and particles that exit glomerular capillaries. Aldosterone promotes the reabsorption of sodium in the DCT. Water reabsorption occurs as a result of the movement of sodium (where sodium goes, water follows). The ability of the kidneys to vary the volume or concentration of urine helps regulate water balance regardless of fluid intake. In this way, the healthy kidney can prevent dehydration when fluid intake is low and can prevent circulatory overload when fluid intake is high. In addition to water, electrolytes are reabsorbed as needed to maintain fluid and electrolyte balance in the blood. Most sodium, chloride, and water reabsorption occurs in the proximal convoluted tubule (PCT). The collecting ducts are the other site of sodium, chloride, and water reabsorption. Here reabsorption is caused by aldosterone. Potassium is mostly reabsorbed in the PCT and thick segment of the loop of Henle. FIG. 60.7 Sodium and water reabsorption by the tubules of a cortical nephron. ADH, Antidiuretic hormone; Na + , sodium. Bicarbonate, calcium, and phosphate are mostly reabsorbed in the PCT. Bicarbonate reabsorption helps acid-base balance and maintains a normal blood pH. Blood levels of calcitonin and parathyroid hormone (PTH) (see Chapters 13 and 58) control calcium balance. Some types of particles in the tubular filtrate are also returned to the blood by tubular reabsorption. About 50% of all urea in the filtrate is reabsorbed; creatinine is not reabsorbed. The kidney reabsorbs some of the glucose filtered from the blood. However, there is a limit to how much glucose the kidney can reabsorb. The point where the kidney is overwhelmed with glucose and can no longer reabsorb is called the renal threshold or transport maximum for glucose reabsorption. The renal threshold for glucose is >180 mg/dL (10 mmol/L). This means that at a blood glucose level of 180 mg/dL (10 mmol/L) or less, all glucose is reabsorbed and returned to the blood, with no glucose present in final urine. When blood glucose levels are greater than 180 mg/dL (10 mmol/L), some glucose stays in the filtrate and is present in the urine (McCulloch, 2018). Normally, almost all glucose and most proteins are reabsorbed and thus are not present in the urine. Nursing Safety Priority Action Alert Report the presence of glucose or proteins in the urine of a patient undergoing a screening examination to the primary health care provider because this is an abnormal finding and requires further assessment. Tubular secretion is the third process of urine formation. It allows substances to move from the blood into the urine. During tubular secretion, substances move from the peritubular capillaries in reverse, across capillary membranes, and into the cells that line the tubules. From the cells, these substances are moved into the urine and excreted from the body. Potassium (K+) and hydrogen (H+) ions are some of the substances moved in this way to maintain fluid and electrolyte balance and acid-base balance (pH). Hormonal Functions The kidneys produce renin, prostaglandins, erythropoietin, and activated vitamin D (Table 60.2). Other kidney products, such as the kinins, change kidney blood flow, regulate blood pressure, and influence capillary permeability. The kidneys also help break down and excrete insulin and many other drugs. Renin, as discussed in the Microscopic Anatomy section, assists in blood pressure control. It is formed and released when there is a decrease in blood flow, blood volume, or blood pressure through the renal arterioles or when too li le sodium is present in kidney blood. These conditions are detected through the receptors of the juxtaglomerular complex. Renin release causes the production of angiotensin II through a series of steps (see Fig. 60.5). Angiotensin II increases systemic blood pressure with powerful blood vessel constricting effects and triggers the release of aldosterone from the adrenal glands. Aldosterone increases the reabsorption of sodium in the distal tubule of the nephron. Therefore more water is reabsorbed, which increases blood volume and blood pressure. When blood flow to the kidney is reduced, this system also prevents fluid loss and maintains circulating blood volume (see Chapter 13). Prostaglandins are produced in the kidney and many other tissues. Those produced specifically in the kidney help regulate glomerular filtration, kidney vascular resistance, and renin production. They also increase sodium and water excretion. Erythropoietin is produced and released in response to decreased oxygen in the kidney’s blood supply. It triggers red blood cell (RBC) production in the bone marrow. When kidney function is poor, erythropoietin production decreases and anemia results. Vitamin D activation occurs through a series of steps. Some of these steps take place in the skin when it is exposed to sunlight, and then more processing occurs in the liver. From there, vitamin D is converted to its active form in the kidney. Activated vitamin D is needed to absorb calcium in the intestinal tract and regulate calcium balance (McCance & Huether, 2019). Ureters Each kidney usually has a single ureter, which is a hollow tube that connects the renal pelvis with the urinary bladder. The ureter is about ½ inch (1.25 cm) in diameter and about 12 to 18 inches (30 to 45 cm) in length. The diameter of the ureter narrows in three areas: • In the upper third of the ureter, at the point at which the renal pelvis becomes the ureter, is a narrowing known as the ureteropelvic junction (UPJ). • The ureter also narrows as it bends toward the abdominal wall (aortoiliac bend). TABLE 60.2 Kidney Hormones and Hormones Influencing Kidney Function CD, Collecting duct; DCT, distal convoluted tubule. • Each ureter narrows at the point at which it enters the bladder; this point is called the ureterovesical junction (UVJ). The ureter tunnels through bladder tissue for a short distance and then opens into the bladder at the trigone (Fig. 60.8). The ureter has three layers: an inner lining of mucous membrane (urothelium), a middle layer of smooth muscle fibers, and an outer layer of fibrous tissue. The middle layer of muscle fibers is controlled by several nerve pathways from the lower spinal cord. Contractions of the smooth muscle in the ureter move urine from the kidney pelvis to the bladder. Stretch receptors in the kidney pelvis regulate this movement. For example, a large volume of urine in the kidney pelvis triggers the stretch receptors, which respond by increasing ureteral contractions and ureter peristalsis. Urinary Bladder Structure The urinary bladder is a muscular sac (see Fig. 60.8) that lies directly behind the pubic bone. In men, the bladder is in front of the rectum. In women, it is in front of the vagina. The bladder is composed of the body (the rounded sac portion) and the bladder neck (posterior urethra), which connects to the bladder body. The bladder has three linings: an inner lining of epithelial cells (urothelium), middle layers of smooth muscle (detrusor muscle), and an outer lining. The trigone is an area on the posterior wall between the points of ureteral entry (ureterovesical junctions [UVJs]) and the urethra. The internal urethral sphincter is the smooth detrusor muscle of the bladder neck and elastic tissue. The external urethral sphincter is skeletal muscle that surrounds the urethra. In men, the external sphincter surrounds the urethra at the base of the prostate gland. In women, the external sphincter is at the base of the bladder. The pudendal nerve from the spinal cord controls the external sphincter. Function The bladder stores urine, provides continence, and enables voiding. The secretions of the urothelium lining the bladder resist bacteria. FIG. 60.8 Gross anatomy of the urinary bladder. Modified from Patton, K.T., & Thibodeau, G.A. [2013]. Anatomy & physiology [8th ed.]. St. Louis: Mosby. Continence is the ability to voluntarily control bladder emptying. It occurs during bladder filling through the combination of detrusor muscle relaxation, internal sphincter muscle tone, and external sphincter contraction. As the bladder fills with urine, stretch sensations are transmi ed to spinal sacral nerves. Maintaining continence occurs by the interaction of the nerves that control the muscles of the bladder, bladder neck, urethra, and pelvic floor, as well as by factors that close the urethra. In the continent person, the smooth muscle of the detrusor remains relaxed during a period of urine filling and storage. Sympathetic nervous system fibers prevent detrusor muscle contraction. The control centers for voiding are located in the cerebral cortex, the brainstem, and the lower spinal cord. For urethral closure to be adequate for continence, the mucosal surfaces must be in contact and must be adhesive. Contact depends on the presence and proper function of the involved nerves and muscles. Adhesion depends on the secretion of mucus-like substances. Micturition (voiding, urination) is a reflex of autonomic control that triggers contraction of the detrusor muscle (closing the ureter at the UVJ to prevent backflow) at the same time as relaxation of the external sphincter and the muscles of the pelvic floor. Voluntary urine elimination (voiding) occurs as a learned response and is controlled by the cerebral cortex and the brainstem. Contraction of the external sphincter inhibits the micturition reflex and prevents voiding. Urethra The urethra is a narrow tube lined with mucous membranes. Its purpose is to allow urine elimination from the bladder. The urethral meatus, or opening, is the endpoint of the urethra. In men, the urethra is about 6 to 8 inches (15 to 20 cm) long, with the meatus located at the tip of the penis. The male urethra has three sections: • The prostatic urethra, which extends from the bladder through the prostate gland • The membranous urethra, which extends from the prostate to the wall of the pelvic floor • The cavernous urethra, which is external and extends through the length of the penis In women, the urethra is 1 to 1.5 inches (2.5 to 3.75 cm) long and exits through the pelvic floor. The meatus lies slightly below the clitoris and directly in front of the vagina and rectum. Kidney and Urinary Changes Associated With Aging Kidney Changes Changes occur in the kidney as a result of the aging process that can affect urine elimination and health (see the Patient-Centered Care: Older Adult Considerations: Changes in the Renal System Related to Aging box). The kidney loses cortical tissue and nephrons and gets smaller with age as a result of reduced blood flow to the kidney (Denic et al., 2016; Touhy & Je , 2016). The medulla is not affected by aging, and the juxtamedullary nephron functions are preserved. The glomerular and tubular linings thicken. Both the number of glomeruli and their surface areas decrease with aging. Tubule length decreases. The changes reduce the older adult’s ability to filter blood and excrete waste products. Patient-Centered Care: Older Adult Considerations Changes in the Renal System Related to Aging Blood flow to the kidney declines by about 10% per decade as blood vessels thicken. This means that blood flow to the kidney is not as adaptive in older adults, leaving nephrons more vulnerable to damage during episodes of either hypotension or hypertension. Glomerular filtration rate (GFR) decreases with age. By age 65 years, the GFR is about 65 mL/min (half the rate of a young adult) and increases the risk for fluid overload. This decline is more rapid in patients with diabetes, hypertension, or heart failure. The combination of reduced kidney mass, reduced blood flow, and decreased GFR contributes to reduced drug clearance and a greater risk for drug reactions and kidney damage from drugs and contrast media in older adults. Tubular changes with aging decrease the ability to concentrate urine, resulting in urgency (a sense of a nearly uncontrollable need to urinate) and nocturnal polyuria (increased urination at night). The regulation of sodium, acids, and bicarbonate is less efficient. Along with an age-related impairment in the thirst mechanism, these changes increase the risk for disturbances of fluid and electrolyte balance, such as dehydration and hypernatremia (increased blood sodium levels) in the older adult. Hormonal changes include a decrease in renin secretion, aldosterone levels, and activation of vitamin D. Urinary Changes Changes in detrusor muscle elasticity lead to decreased bladder capacity and reduced ability to retain urine (Touhy & Je , 2016). The urge to void may cause immediate bladder emptying because the urinary sphincters lose tone and often become weaker with age. In women, weakened muscles in the pelvic floor shorten the urethra and promote incontinence. In men, an enlarged prostate gland makes starting the urine stream difficult and may cause urinary retention. Patient-Centered Care: Cultural/Spiritual Considerations African Americans have more rapid age-related decreases in GFR than do white adults. Kidney excretion of sodium is less effective in hypertensive African Americans who have high sodium intake, and the kidneys have about 20% less blood flow as a result of anatomic changes in small blood vessels and intrarenal responses to renin. Thus African-American patients are at greater risk for kidney failure than are white patients (Jarvis, 2020). Yearly health examinations should include urinalysis, checking for the presence of microalbuminuria, and evaluating serum creatinine. Assessment: Recognize Cues Patient History Demographic information, such as age, gender, race, and ethnicity, is important to consider as nonmodifiable risk factors in the patient with any kidney or urinary elimination problem. A sudden onset of hypertension in patients older than 50 years suggests possible kidney disease. Clinical changes in polycystic kidney disease typically occur in patients in their 40s or 50s. In men older than 50 years, altered urine pa erns accompany prostate disease. Anatomic gender differences make some disorders worse or more common. For example, men rarely have ascending urinary tract infections. Women have a shorter urethra and more commonly develop cystitis (bladder inflammation, most often with infection) because bacteria pass more readily into the bladder. Modifiable risk factors, as well as socioeconomic status, level of education, language, and health beliefs, should be considered when assessing renal function. Socioeconomic status may influence health care practices. Prevention, early detection, and treatment of kidney or urinary problems may be limited by inability to access to health care, lack of transportation, insufficient or no insurance, and/or reduced income. These barriers may also result in difficulty following medical advice, having prescriptions filled, adhering to dietary instructions, and keeping followup appointments. Educational level may affect health-seeking practices and the patient’s understanding of a disease or its symptoms. Recurring urinary tract infections can result from not completing a course of antibiotic therapy or from not following up to ensure that the infection is cleared. The language used by patients may be different from that used by the health care professional. When obtaining a history, listen to and explore the terms used by the patient. By using the patient’s own terms, you may help him or her provide a more complete description of the problem and may decrease the patient’s discomfort when discussing bodily functions. The patient’s health beliefs affect the approach to health and illness. Cultural background or religious affiliation may influence the belief system, as well as comfort when discussing issues about elimination (Jackson et al., 2013). Ask the patient about previous kidney or urologic problems, including tumors, infections, stones, or urologic surgery. A history of any chronic health problems, especially diabetes mellitus or hypertension, increases the risk for development of kidney disease because these disorders damage kidney blood vessels. Ask the patient about environmental, food, or medication allergies. Exposure to certain contrast media during imaging can harm the kidneys. Iodinated contrast medium used for CT scans is associated with both acute and chronic kidney injury (Lambert et al., 2017). High-osmolarity contrast agents can also contribute to kidney function impairment. Exposure to gadolinium-enhanced MRI can result in nephrogenic systemic fibrosis. Ask the patient about chemical exposures at the workplace or with hobbies. Exposure to hydrocarbons (e.g., gasoline, oil), heavy metals (especially mercury and lead), and some gases (e.g., chlorine, toluene) can impair kidney function. Use this opportunity to teach patients who come into contact with chemicals at work or during leisure-time activities to avoid direct skin or mucous membrane contact with these chemicals. Use of heroin, cocaine, methamphetamine, ecstasy, and volatile solvents (inhalants) has also been associated with kidney damage. Specifically ask the patient whether he or she has ever been told about the presence of protein or albumin in the urine. The question, “Have you ever been told that your blood pressure is high?” may prompt a response different from the one to the question, “Do you have high blood pressure?” Ask women about health problems during pregnancy (e.g., proteinuria, high blood pressure, gestational diabetes, urinary tract infections). Obtain information about: • Chemical or environmental toxin exposure in occupational, diagnostic, or other se ings • Recent travel to geographic regions that pose infectious disease risks • Recent trauma or injury, particularly to the abdomen or pelvic or genital areas • A history of altered pa erns of urinary elimination Nutrition History Ask the patient with known or suspected kidney or urologic disorders about diet and any recent dietary changes. Note any excessive intake or omission of certain food categories. Ask about food and fluid intake. Assess how much and which types of fluids the patient drinks daily, especially fluids with a high-calorie or caffeine content. Use this opportunity to teach the patient the importance of drinking sufficient fluid to cause urine to be dilute (clear or very light yellow). If another medical problem does not require fluid restriction, ingestion of about 2 L of fluid daily is recommended. If the patient has followed a diet for weight reduction, the details of the diet plan are important and collaboration with a dietitian may be needed. A high-protein intake can result in temporary kidney problems. For example, a patient at risk for calculi (stone) formation who ingests large amounts of protein or has a poor fluid intake may form new stones. Ask about any change in appetite or taste. These symptoms can occur with the buildup of nitrogenous waste products from kidney failure. Changes in thirst or fluid intake may also cause changes in the volume of urine elimination. Endocrine disorders may also cause changes in thirst, fluid intake, and urine output. (See Chapter 56 for a discussion of endocrine influences on fluid balance.) Medication History Identify all of the patient’s prescription drugs because many can impair kidney function (Burchum & Rosenthal, 2019). Ask about the duration of drug use and whether there have been any recent changes in prescribed drugs. Drugs for diabetes mellitus, hypertension, cardiac disorders, hormonal disorders, cancer, arthritis, and psychiatric disorders are potential causes of kidney problems. Antibiotics, such as gentamicin, may also cause acute kidney injury. Drug-drug interactions and drug–contrast media interactions also may lead to kidney dysfunction (Lambert et al., 2017). Explore the past and current use of over-the-counter (OTC) drugs or agents, including dietary supplements, vitamins and minerals, herbal agents, laxatives, analgesics, acetaminophen, and NSAIDs. Many of these agents affect kidney function and urine elimination. For example, dietary supplementation with synthetic creatine, used to increase muscle mass, has been associated with compromised kidney function. High-dose or long-term use of NSAIDs or acetaminophen can seriously reduce kidney function. Some agents are associated with hypertension, hematuria, or proteinuria, which may occur before kidney dysfunction. Family History and Genetic Risk The family history of the patient with a suspected kidney or urologic problem is important because some disorders have a familial pa ern. Ask whether siblings, parents, or grandparents have had kidney problems. Past terms used for kidney disease include Bright disease, nephritis, and nephrosis. Although nephritis is a current term for an inflammatory process in the kidney and nephrosis is a current term for a degenerative process in the kidney, these terms have been used by lay adults for years to describe any type of kidney problem. Polycystic kidney disease, which is a genetic disorder, can occur in either gender. Current Health Problem The effects of kidney failure are seen in all body systems. Document all of the patient’s current health problems. Ask the patient to describe all health concerns, because some kidney disorders cause problems in other body systems. Recent upper respiratory problems, achy muscles or joints, heart disease, or GI conditions may be related to problems of kidney function. Assess the kidney and urologic system by asking about any changes in the appearance (color, odor, clarity) of the urine, pa ern of urine elimination, ability to initiate or control voiding, and other unusual symptoms. For example, urine that is reddish, rust-colored, brown or black, greenish, or different from the usual yellowish color may prompt the patient to seek health care assistance. Urine typically has a mild but distinct odor of ammonia. An increase in the intensity of color, a change in odor quality, or a decrease in urine clarity may suggest infection. Ask about changes in urination pa erns, such as incontinence (involuntary bladder emptying), nocturia (urination at night), urgency (nearly uncontrollable urge to urinate), frequency, or an increase or decrease in the amount of urine. The normal urine output for adults is about 1500 to 2000 mL/day or within 500 mL of the volume of fluid ingested daily. Ask about how closely the urine output is to the volume of fluid ingested. A bladder diary may be useful. Ask whether: • Initiating urine flow is difficult • A burning sensation or other discomfort occurs with urination • The force of the urine stream is decreased • Persistent dribbling or leaking of urine is present The onset of pain in the flank, in the lower abdomen or pelvic region, or in the perineal area triggers concern and usually prompts the patient to seek assistance. Ask about the onset, intensity, and duration of the pain; its location; precipitating and relieving factors; and its association with any activity or event. Pain associated with kidney or ureteral irritation is often severe and spasmodic. Pain that radiates into the perineal area, groin, scrotum, or labia is described as renal colic. This pain occurs with distention or spasm of the ureter, such as in an obstruction or the passing of a stone. Renal colic pain may be intermi ent or continuous and may occur with pallor, diaphoresis, and hypotension. These general symptoms occur because of the location of the nerve tracts near or in the kidneys and ureters (Brenner, 2016). Because the kidneys are close to the GI organs and the nerve pathways are similar, GI symptoms may occur with kidney problems. These renointestinal reflexes often complicate the description of the kidney problem. Uremia is the buildup of nitrogenous waste products in the blood from inadequate elimination as a result of kidney failure. Symptoms include anorexia, nausea and vomiting, muscle cramps, pruritus (itching), fatigue, and lethargy. Physical Assessment The physical assessment of the patient with a known or suspected kidney or urologic disorder includes general appearance, a review of body systems, and specific structure and functions of the kidney and urinary system. Assess the patient’s general appearance and check the skin for the presence of any rashes, bruising, or yellowish discoloration. The skin and tissues may show edema associated with kidney disease, especially in the pedal (foot), pretibial (shin), and sacral tissues and around the eyes. Use a stethoscope to listen to the lungs to determine whether fluid is present. Weigh the patient and measure blood pressure as a baseline for later comparisons. Assess the levels of consciousness and alertness. Record any deficits in memory, concentration, or thought processes. Family members may report subtle changes. Cognitive changes may be the result of the buildup of waste products when kidney disease is present. Assessment of the Kidneys, Ureters, and Bladder Assess the kidneys, ureters, and bladder during an abdominal assessment (Jarvis, 2020). Auscultate before percussion and palpation because these activities can alter bowel sounds and obscure abdominal vascular sounds. Inspect the abdomen and the flank regions with the patient in both the supine and si ing positions. Observe the patient for asymmetry (e.g., swelling) or discoloration (e.g., bruising or redness) in the flank region, especially in the area of the costovertebral angle (CVA). The CVA is located between the lower portion of the twelfth rib and the vertebral column. Listen for a bruit by placing a stethoscope over each renal artery on the midclavicular line. A bruit is an audible swishing sound produced when the volume of blood or the diameter of the blood vessel changes. It often occurs with blood flow through a narrowed vessel, as in renal artery stenosis. Kidney palpation is usually performed by a health care provider. It can help locate masses and areas of tenderness in or around the kidney. The health care provider will lightly palpate the abdomen in all quadrants, ask about areas of tenderness or pain, and examine nontender areas first (Fig. 60.9). The outline of the bladder may be noted as high as the umbilicus in patients with severe bladder distention. Nursing Safety Priority Action Alert Performing palpation on a patient with a suspected abdominal tumor or aneurysm may harm the patient. Because the kidneys are located deep and posterior, palpation is easier in thin patients who have li le abdominal musculature. For palpation of the right kidney, the patient is placed in a supine position while the examiner places one hand under the right flank and the other hand over the abdomen below the lower right part of the rib cage. The lower hand is used to raise the flank, and the upper hand depresses the abdomen as the patient takes a deep breath (Fig. 60.9). The left kidney is deeper and often cannot be palpated. A transplanted kidney is readily palpated in either the lower right or left abdominal quadrant. The normal kidney is smooth, firm, and nontender. FIG. 60.9 Advanced technique for palpation of the kidney. A distended bladder sounds dull when percussed. After gently palpating to determine the outline of the distended bladder, begin percussion on the lower abdomen and continue in the direction of the umbilicus until dull sounds are no longer produced. If you suspect bladder distention, use a portable bladder scanner (Fig. 60.10) to determine the amount of retained urine. If the patient reports flank pain or tenderness, the nontender flank should be percussed first. For percussion, the patient is placed in a si ing, side-lying, or supine position. Percussion, generally performed by the health care provider, is done by forming one hand into a clenched fist and the other hand lies flat over the CVA of the patient. Using the hand in a fist, a quick, firm thump is administered to the hand over the CVA area (Jarvis, 2020). Costovertebral tenderness often occurs with kidney infection or inflammation. Patients with inflammation or infection in the kidney or nearby structures may describe their pain as severe or as a constant, dull ache. Assessment of the Urethra Using a good light source and wearing gloves, inspect the urethra by examining the meatus and the tissues around it. Record any unusual discharge such as blood, mucus, or pus. Inspect the skin and mucous membranes of surrounding tissues. Record the presence of lesions, rashes, or other abnormalities of the penis or scrotum or of the labia or vaginal opening. Urethral irritation is suspected when the patient reports discomfort with urination. Use this opportunity to remind women to clean the perineum by wiping from front to back, never from back to front. Teach them that the front-to-back technique keeps organisms in stool from coming close to the urethra and decreases the risk for infection. FIG. 60.10 “BladderScan” BVI 9400, a handheld portable bladder scanner. Courtesy Verathon Corporation, Bothell, WA. Patient-Centered Care: Cultural/Spiritual Considerations Women from some cultures or religions may have undergone female circumcision. This procedure alters the appearance of the vulvar-perineal area and increases the risk for urinary tract infections. It also makes urethral inspection or catheterization difficult. Document any noted anatomic changes and ask the patient to describe hygiene practices for this area. Psychosocial Assessment Concerns about the urologic system may evoke fear, anger, embarrassment, anxiety, guilt, or sadness in the patient. Childhood learning often includes the idea that toileting should take place in private and not be discussed with other people. Urologic disorders may bring up forgo en memories of difficult toilet training and bedwe ing or of childhood experiences of exploring one’s body. The patient may ignore symptoms or delay seeking health care because of emotional responses or cultural taboos about the urogenital area. NCLEX Examination Challenge 60.1 Physiological Integrity When obtaining a health history and physical assessment from a 68-yearold male client who has a history of an enlarged prostate, which finding does the nurse consider significant? Select all that apply. A. Distended bladder B. Absence of a bruit C. Frequency of urination D. Dribbling urine after voiding E. Chemical exposure in the workplace Diagnostic Assessment Laboratory Assessment Blood Tests Serum creatinine is produced when muscle and other proteins are broken down. Because protein breakdown is usually constant, the serum creatinine level is a good indicator of kidney function. Serum creatinine levels are slightly higher in men than in women because men tend to have a larger muscle mass than do women. Similarly, adults with greater muscle mass or muscle mass turnover (e.g., athletes) may have a slightly higher-than-average serum creatinine level. Muscle mass and the amount of creatinine produced decrease with age. However, because of decreased rates of creatinine clearance, the serum creatinine level remains relatively constant in older adults unless kidney disease is present. No common pathologic condition other than kidney disease increases the serum creatinine level. When the serum creatinine level is doubled, it indicates a 50% reduction in glomerular filtration rate (Pagana & Pagana, 2018); therefore any elevation of serum creatinine values is important and should be assessed further. Creatinine is excreted solely by the kidneys. Nursing Safety Priority Action Alert A serum creatinine of 1.5 mg/dL (110 mcmol/L) or greater places a patient at risk for acute kidney injury (AKI) from iodinated contrast media and some drugs (Lambert et al., 2017). Monitor both baseline and trend values to recognize risk for and actual kidney damage, especially among patients exposed to agents that can cause kidney dysfunction. If indicated, respond by promptly informing the primary health care provider of increases in serum creatinine greater than 1.5 times the baseline and urine output values of less than 0.5 mL/kg/hr for 6 or more hours. Using the baseline and trending creatinine levels is important, especially in older adults and young children as they have lower creatinine levels due to reduced muscle mass than the normal adult. Blood urea nitrogen (BUN) measures the effectiveness of kidney excretion of urea nitrogen, a by-product of protein breakdown in the liver. Urea nitrogen is produced mostly from liver metabolism of food sources of protein. The kidneys filter urea nitrogen from the blood and excrete the waste as part of urine elimination. Other factors influence the BUN level, and an elevation does not always mean that kidney disease is present (see the Laboratory Profile: Kidney Function Blood Studies box). For example, rapid cell destruction from infection, cancer treatment, or steroid therapy may elevate BUN level. In addition, blood is a protein. Blood in the tissues rather than in the blood vessels is reabsorbed as if it were a general protein. Thus reabsorbed blood protein is processed by the liver and increases BUN levels. This means that injured tissues can result in increased BUN levels even when kidney function is normal. In addition, BUN is increased by protein turnover in exercising muscle and is elevated as a result of concentration during dehydration. The liver must function properly to produce urea nitrogen. When liver and kidney dysfunction are present, urea nitrogen levels are actually decreased because the liver failure limits urea production. The BUN level is not always elevated with kidney disease and is not the best indicator of kidney function. However, an elevated BUN level suggests kidney dysfunction. Blood urea nitrogen to serum creatinine ratio can help determine whether non–kidney-related factors, such as low cardiac output or red blood cell destruction, are causing the elevated BUN level. When blood volume is deficient (e.g., dehydration) or cardiac output is low, the BUN level rises more rapidly than the serum creatinine level. As a result, the ratio of BUN to creatinine is increased. When both the BUN and serum creatinine levels increase at the same rate, the BUN/creatinine ratio remains normal. However, elevations of both serum creatinine and BUN levels suggest kidney dysfunction that is not related to dehydration or poor perfusion. Cystatin-C measures glomerular filtration rate. Cystatin-C is a protein produced by nucleated cells in the body. Since cystatin-C is produced at a constant rate, it can be used as an indicator of glomerular filtration rate. When the glomerular filtration rate is reduced, cystatin-C increases. Increased levels can be considered a predictor of chronic renal disease. Cystatin-C is not influenced by factors that influence BUN and creatinine levels, making it potentially a be er indicator of glomerular filtration rate. Research is still in progress as to the efficacy of cystatin-C in the role of identifying renal disease and other health alterations such as cardiovascular disease and metabolic syndrome (Pagana & Pagana, 2018). Blood osmolarity is a measure of the overall concentration of particles in the blood and is a good indicator of hydration status. The kidneys excrete or reabsorb water to keep blood osmolarity in the range of 280 to 300 mOsm/kg (mmol/kg). Osmolarity is slightly higher in older adults. When blood osmolarity is decreased, vasopressin (antidiuretic hormone [ADH]) y p release is inhibited. Without vasopressin, the distal tubule and collecting ducts are not permeable to water. As a result, water is excreted, not reabsorbed, and blood osmolarity increases. When blood osmolarity increases, vasopressin is released. Vasopressin increases the permeability of the distal tubule to water. Then water is reabsorbed, and blood osmolarity decreases. Urine Tests Urinalysis Urinalysis is a part of any complete physical examination and is especially useful for patients with suspected kidney or urologic disorders (see the Laboratory Profile: Urinalysis box). Ideally, the urine specimen is collected at the morning’s first voiding. Specimens obtained at other times may be too dilute. The specimen may be collected by several techniques (Box 60.1). Urine color comes from urochrome pigment. Color variations may result from increased levels of urochrome or other pigments, changes in the concentration or dilution of the urine, and the presence of drug metabolites in the urine. Urine smells faintly like ammonia and is normally clear without turbidity (cloudiness) or haziness. Specific gravity is the concentration of particles (i.e., electrolytes, wastes) in urine. A high specific gravity indicates concentrated urine from dehydration, decreased kidney blood flow, or excess vasopressin associated with stress, surgery, anesthetic agents, and certain drugs (e.g., morphine, some oral antidiabetic drugs) or syndrome of inappropriate antidiuretic hormone (SIADH) (see Chapter 57). Low specific gravity indicates dilute urine that may occur from high fluid intake, diuretic drugs, or diabetes insipidus (DI) (see Chapter 57). Specific gravity of urine is compared with distilled water, which has a specific gravity of 1.000. The normal specific gravity of urine ranges from 1.005 to about 1.030. Kidney disease diminishes the concentrating ability of the kidney, and chronic kidney disease may be associated with a low (dilute) specific gravity. pH is a measure of urine acidity or alkalinity. A pH value less than 7 is acidic, and a value greater than 7 is alkaline. Urine pH is affected by diet, drugs, systemic disturbances of acid-base balance, and kidney tubular function. For example, a high-protein diet produces acidic urine, whereas a high intake of citrus fruit produces alkaline urine. The normal pH of urine ranges from 4.6 to 8.0 with an average of 6.0 (Pagana & Pagana, 2018). Urine specimens become more alkaline when left standing unrefrigerated for more than 1 hour, when bacteria are present, Laboratory Profile Kidney Function Blood Studies Data from Pagana, K., & Pagana, T. (2018). Mosby’s manual of diagnostic & laboratory test (6th ed.). St. Louis: Mosby; and Pagana, K., Pagana, T., & Pike-McDonald, S. (2018). Mosby’s Canadian manual of diagnostic and laboratory tests. St. Louis: Elsevier. Laboratory Profile Urinalysis Test Color Odor Turbidity Specific gravity Normal Range for Adults Yellow Specific aroma, similar to ammonia Clear 1.005-1.030; usually 1.010-1.025 Older adult: decrease with age pH Average: 6; range: 4.6-8 Glucose Fresh specimen, negative 50-300 mg/day in a 24-hr specimen None Ketones Significance of Abnormal Findings Dark amber indicates concentrated urine. Very pale yellow indicates dilute urine. Dark red or brown indicates blood in the urine. Brown may indicate increased bilirubin level. Red also may indicate the presence of myoglobin. Other color changes may result from diet or drugs. Foul smell indicates possible infection, dehydration, or ingestion of certain foods or drugs. Cloudy urine indicates infection, sediment, or high levels of urine protein. Increased in decreased kidney perfusion, inappropriate ADH secretion, or heart failure. Decreased in chronic kidney disease, diabetes insipidus, malignant hypertension, diuretic administration, and lithium toxicity. Changes are caused by diet, drugs, infection, age of specimen, acid-base imbalance, and kidney disease. Presence reflects hyperglycemia or a decrease in the kidney threshold for glucose. Presence occurs with diabetic ketoacidosis, prolonged fasting, and anorexia nervosa. Test Protein Bilirubin (urobilinogen) Normal Range for Adults 0-8 mg/dL (5080 mg in 24-hr specimen at rest <250 mg in 24-hr specimen with exercise None Significance of Abnormal Findings Increased amounts may indicate stress, infection, recent strenuous exercise, or glomerular disorders. Presence suggests liver or biliary disease or obstruction. Red blood cells (RBCs) 0-2 per highpower field White blood cells (WBCs) 0-4 per lowpower field Casts None Crystals None Bacteria <1000 colonies/mL Parasites None Leukocyte esterase None Increased is normal with catheterization or menses but may reflect tumor, stones, trauma, glomerular disorders, cystitis, or bleeding disorders. Increased may indicate an infection or inflammation in the kidney and urinary tract, kidney transplant rejection, or exercise. Increased indicates bacteria, protein, or urinary calculi. Presence may indicate that the specimen has been allowed to stand. Increased indicates the need for urine culture to determine the presence of urinary tract infection. Presence of Trichomonas vaginalis indicates infection, usually of the urethra, prostate, or vagina. Presence suggests urinary tract infection. Nitrites None Presence suggests urinary Escherichia coli. Data from Pagana, K., & Pagana, T. (2018). Mosby's manual of diagnostic & laboratory test (6th ed.). St. Louis: Mosby; and Pagana, K., Pagana, T., & Pike-McDonald, S. (2018). Mosby's Canadian manual of diagnostic and laboratory tests. St. Louis: Elsevier. or when a specimen is left uncovered. Alkaline urine increases cell breakdown; thus the presence of red blood cells may be missed on analysis. Ensure that urine specimens are covered and delivered to the laboratory promptly. Urine specimens delayed 2 or more hours require refrigerated or other specific storage and transport precautions to ensure the integrity of the urine specimen (Pagana & Pagana, 2018). During systemic acidosis or alkalosis, the kidneys, along with blood buffers and the lungs, normally respond to keep serum pH normal. Chapter 14 discusses acid-base balance and imbalance. Protein is not normally present in the urine. Microalbumin levels greater than 80 mcg/24 hr (0.08 g/24 hr) are abnormal. Protein molecules are too large to pass through intact glomerular membranes. When glomerular membranes are not intact, protein molecules pass through and are excreted with urine elimination. Increased membrane permeability is caused by infection, inflammation, or immunologic problems. Some systemic problems cause production of abnormal proteins, such as globulin. Detection of abnormal protein types requires electrophoresis. A random finding of proteinuria (usually albumin in the urine) followed by a series of negative (normal) findings does not imply kidney disease. If infection is the cause of the proteinuria, urinalyses after resolution of the infection should be negative for protein. Persistent proteinuria needs further investigation. Microalbuminuria is the presence of albumin in the urine that is not measurable by a urine dipstick or usual urinalysis procedures. Specialized assays are used to quickly analyze a freshly voided urine specimen for microscopic levels of albumin. The normal microalbumin levels in a freshly voided specimen should be less than 2.0 mg/dL. Higher levels indicate microalbuminuria and could mean mild or early kidney disease, especially in patients with diabetes mellitus. In 24-hour urine specimens, levels greater than 80 mcg/24 hr (0.08 g/24 hr) indicate microalbuminuria. B O X 6 0 . 1 Co llectio n o f U r ine Specim ens Glucose in the urine may indicate a high level of glucose in the blood, typically greater than 220 mg/dL (12 mmol/L). Changes in the renal threshold for glucose may occur temporarily in patients who have infection or severe stress. Ketone bodies are formed from the incomplete metabolism of fa y acids. Three types of ketone bodies are acetone, acetoacetic acid, and betahydroxybutyric acid. Normally there are no ketones in urine. Ketone bodies are produced when fat is used instead of glucose for cellular energy. Ketones present in the blood are partially excreted in the urine. Leukoesterase is an enzyme found in some white blood cells, especially neutrophils. When the number of these cells increases in the urine or they are damaged (lysed), the urine then contains leukoesterase. A normal reading is no leukoesterase in the urine. A positive test (+ sign) is an indication of a urinary tract infection. Nitrites are not usually present in urine. Many types of bacteria, when present in the urine, convert nitrates (normally found in urine) into nitrites. A positive nitrites test enhances the sensitivity of the leukoesterase test to detect urinary tract infection (Pagana & Pagana, 2018). Sediment is precipitated particles in the urine. These particles include cells, casts, crystals, and bacteria. Normally, urine contains few, if any, cells. Types of cells abnormally present in the urine include tubular cells (from the tubule of the nephron), epithelial cells (from the lining of the urinary tract), red blood cells (RBCs), and white blood cells (WBCs). WBCs may indicate a urinary tract or kidney infection. RBCs may indicate glomerulonephritis, acute tubular necrosis, pyelonephritis, kidney trauma, or kidney cancer. Casts are clumps of materials or cells. When cells, bacteria, or proteins are present in the urine, minerals and sticky materials clump around them and form a cast of the distal renal tubule and collecting duct. Casts are described by the type of particle they have surrounded (e.g., hyaline [protein-based] or cellular [from RBCs, WBCs, or epithelial cells]) or the stage of cast breakdown (whole cell or granular from cell breakdown). Although an isolated urinalysis with sediment from casts may be the result of strenuous exercise, repeated findings with sediment are more likely to be associated with disease. Urine crystals come from mineral salts as a result of diet, drugs, or disease. Common salt crystals are formed from calcium, oxalate, urea, phosphate, magnesium, or other substances. Some drugs, such as the sulfates, can also form crystals. Crystals can form into calculi. y y Bacteria multiply quickly, so the urine specimen must be analyzed promptly to avoid falsely elevated counts of bacterial colonization. Normally urine is sterile, but it can be easily contaminated by perineal bacteria during collection. Recent advances in technology and molecular biology have led to new diagnostic tests using urine, including identification of biomarkers of disease and profiling for specific proteins. Markers such as cystatin-C are being investigated to identify early-onset kidney dysfunction, target therapy, and predict responsiveness to intervention. Other markers for angiogenesis and kidney cell adhesion, regulation, and apoptosis (i.e., connective tissue growth factor [CTGF], neutrophil gelatinase-associated lipocalin [NGAL]) will likely contribute to clinical diagnostics in the future. Urine for Culture and Sensitivity Urine is analyzed for the number and types of organisms present. Symptoms of infection and unexplained bacteria in a urine specimen are indications for urine culture and sensitivity testing. Bacteria from urine are placed in a medium with different antibiotics. In this way, we can know which antibiotics are effective in killing or stopping the growth of the organisms (organisms are “sensitive”) and which are not effective (organisms are “resistant”). A clean-catch or catheter-derived specimen is best for culture and sensitivity testing as these procedures reduce the chance of perineal surface organisms contaminating the specimen. Composite Urine Collections Some urine collections are made for a specified number of hours (e.g., 24 hours) for precise analysis of urine levels of substances, such as creatinine or urea nitrogen, sodium, chloride, calcium, catecholamines, or other components (see the Laboratory Profile: 24-Hour Urine Collection box). For a composite urine specimen, all urine within the designated time frame must be collected. If other urine must be obtained while the collection is in progress, measure and record the amount collected but not added to the timed collection. The urine collection may need to be refrigerated or stored on ice to prevent changes in the urine during the collection time. Follow the procedure from the laboratory for urine storage, including whether a preservative is to be added. The urine collection must be free from fecal contamination. Menstrual blood and toilet tissue also contaminate the specimen and can invalidate the results. The collection of all urine for a 24-hour period is often challenging. With hospitalized patients, the cooperation of staff personnel, the patient, family members, and visitors is essential. Placing signs in the bathroom, instructing the patient and family, and emphasizing the need to save the urine are helpful. Creatinine Clearance Creatinine clearance is a measure of glomerular filtration rate (GFR) and kidney function. The patient’s age, gender, height, weight, diet, and activity level influence the expected amount of excreted creatinine. Thus these factors are considered when interpreting creatinine clearance test results. Decreases in the creatinine clearance rate may require reducing drug doses and often signifies the need to further explore the cause of kidney deterioration. Commonly, creatinine clearance is calculated from serum creatinine, age, weight, urine creatinine, gender, and race. Creatinine clearance can be based on the excretion of injected inulin or other substances that are not reabsorbed into the blood. Creatinine clearance to estimate GFR can also be based on a 24-hour urine collection, although urine can be collected for shorter periods (e.g., 8 or 12 hours). The analysis compares the urine creatinine level with the blood creatinine level; therefore a blood specimen for creatinine must also be collected. The range for normal creatinine clearance is 107 to 139 mL/min for men (1.78 to 2.32 mL/sec) and 87 to 107 mL/min (1.45 to 1.78 mL/sec) for women tested with a 24-hour urine collection (Pagana & Pagana, 2018). Values decrease Laboratory Profile 24-Hour Urine Collection Data from Pagana, K., & Pagana, T. (2018). Mosby’s manual of diagnostic & laboratory test (6th ed.). St. Louis: Mosby; Pagana, K., Pagana, T., & Pike-McDonald, S. (2018). Mosby’s Canadian manual of diagnostic and laboratory tests. St. Louis: Elsevier; and United States Library of Medicine. progressively per decade of life for adults older than 40 years because of age-related decline in GFR. However, these expensive and timeconsuming methods are usually reserved for when a decision for starting renal replacement therapy (dialysis) is needed. Current guidelines suggest that clinical laboratories report an estimate of GFR (eGFR) based on the Modification of Diet in Renal Disease (MDRD) study equation. The MDRD equation does not require urine to estimate GFR; the calculation requires the serum creatinine level, age, and numbers specific to gender and ethnicity. The calculation is an accurate way to measure urine creatinine clearance. The estimated GFR (eGFR) for the MDRD equation is >60 mL/min/1.73 m2 (Pagana & Pagana, 2018). Urine Electrolytes Urine samples can be analyzed for electrolyte levels (e.g., sodium, chloride). Normally the amount of sodium excreted in the urine is nearly equal to that consumed. Urine sodium levels can vary depending on the amount of water and salt consumed. Normal values for a 24-hour urine sample ranges from 40 to 220 mEq/day (or 40 to 220 mmol/day). A value of greater than 20 mEq/L for a routine urine specimen is considered normal (Pagana & Pagana, 2018). NCLEX Examination Challenge 60.2 Physiological Integrity A client is on a 24-hour urine collection. At midpoint during the collection, the client tells the nurse that some of the urine was discarded. What action will the nurse take? Select all that apply. A. No action is required. B. Reinforce client education. C. Notify the laboratory staff. D. Restart the urine collection. E. Document the discarded urine. F. Notify the health care provider. Urine Osmolarity Osmolarity measures the concentration of particles in solution. The particles in urine contributing to osmolarity include electrolytes, glucose, urea, and creatinine. Urine osmolarity can vary from 50 to 1200 mOsm/kg or L (mmol/kg or L), depending on the patient’s hydration status and kidney function. With average fluid intake, the range for urine osmolarity is 300 to 900 mOsm/kg or L (mmol/kg or L). Electrolytes, acids, and other normal metabolic wastes are continually produced. These particles are the solute load that must be excreted in the urine on a regular basis. This is referred to as obligatory solute excretion. If the patient loses excessive fluids, the kidney response is to save water while excreting wastes by excreting small amounts of highly concentrated urine. Diet, drugs, and activity can change urine osmolarity. Urine with an increased osmolarity is concentrated urine with less water and more solutes. Urine with a decreased osmolarity is dilute urine with more water and fewer solutes. Bedside Sonography/Bladder Scanners The use of portable ultrasound scanners in the hospital and rehabilitation se ing by nurses is a noninvasive method of estimating bladder volume (see Fig. 60.10). Bladder scanners are used to screen for postvoid residual volumes and determine the need for intermi ent catheterization based on the amount of urine in the bladder rather than the time between catheterizations. There is no discomfort with the scan, and no patient preparation beyond an explanation of what to expect is required. Explain the reason the procedure is being done and what sensations the patient might experience during the procedure. For example, “This test will measure the amount of urine in your bladder. I will place a gel pad just above your pubic area and then place the probe, which is a li le bigger and heavier than a stethoscope, on the gel.” Before scanning, select the male or female icon on the bladder scanner. Using the female icon allows the scanner software to subtract the volume of the uterus from any measurement. Use the male icon on all men and on women who have undergone a hysterectomy. Place an ultrasound gel pad right above the pubic bone or moisten the round dome of the scan head area with 5 mL of conducting gel to improve ultrasound conduction. Use gel on the scanner head for obese patients and those with heavy body hair in the area to be scanned. Place the probe midline over the abdomen about 1.5 inches (4 cm) above the pubic bone. Aim the scan head so the ultrasound is projected toward the expected location of the bladder, typically toward the patient’s coccyx. Press and release the scan bu on. The scan is complete with the sound of a beep, and a volume is displayed. Two readings are recommended for best accuracy. An aiming icon on the portable bladder scanner indicates whether the bladder image is centered on the crosshairs of the scan head. If the crosshairs on the aiming icon are not centered on the bladder, the measured volume may not be accurate. Imaging Assessment Many imaging procedures are used to diagnose abnormalities within the renal-urinary system (Box 60.2). Explain the procedures, prepare, and provide follow-up care to the patient. Patient education materials for many urologic tests have been developed by organizations, such as the Society for Urologic Nurses and Associates, and are freely available. Encourage the patient to use reliable and credible sources for online information. Kidney, Ureter, and Bladder X-rays An x-ray of the kidneys, ureters, and bladder (KUB) is a plain film of the abdomen obtained without any specific patient preparation. The KUB study shows gross anatomic features and obvious stones, strictures, calcifications, or obstructions in the urinary tract. This test identifies the shape, size, and position of the organs in relation to other parts of the urinary tract. Other tests are needed to diagnose functional or structural problems. There is no discomfort or risk from this procedure. Tell the patient that the x-ray will be taken while in a supine position. No specific follow-up care is needed. B O X 6 0 . 2 R adio lo g ic and Special Diag no stic Tests f o r Pa tie nts With Diso r der s o f the K idney and U r inar y Sy stem Test Radiography of kidneys, ureters, and bladder (KUB) (plain film of abdomen) Purpose To screen for the presence of two kidneys To measure kidney size To detect gross obstruction in kidneys or urinary tract Computed tomography (CT) with contrast, CT arteriography or angiography To measure kidney size To evaluate contour to assess for injury, masses, or obstruction in kidneys or the urinary tract To assess renal blood flow Magnetic resonance imaging (MRI) Similar to CT Useful for staging of cancers Ultrasonography (US) Can be used with contrast media (Nuclear) renal scan Cystoscopy Cystography and cystourethrography With or without retrograde studies With or without contrast medium Metabolic imaging with positron emission tomography (PET) To identify the urine volume in the bladder, size of the kidneys or obstruction (e.g., tumors, stones) in the kidneys or lower urinary tract Assess blood flow to and from the kidney To evaluate renal perfusion To estimate glomerular filtration rate To provide functional information without exposing the patient to iodinated contrast medium To identify abnormalities of the bladder wall and urethral and ureteral occlusions To treat small obstructions or lesions via fulguration, lithotripsy, or removal with a stone basket To outline bladder’s contour when full and examine structure during voiding To examine the structure of the urethra To detect backward urine flow To evaluate cysts, tumors, and other lesions, eliminating the need for biopsy in some patients Computed Tomography Inform the patient that a CT scan provides three-dimensional information about the kidneys, ureters, bladder, and surrounding tissues. The CT scan is performed in a special room, usually in the radiology department. It can provide information about tumors, cysts, abscesses, other masses, and obstruction. CT can also be used to image the kidney’s vascular system (i.e., CT angiography). Some hospitals require patients having CT scans to be NPO for some period before the scan, although there is no specific evidence guiding this practice. Determine whether the scan requires contrast medium (often called dye). The most common contrast agents used for imaging of the kidney are radiopaque, contain iodine, are nonionic, and have varying osmolarity. These include iohexol, iopromide, and iodixanol. Oral or injected contrast medium is usually given before starting the imaging procedure. Dye use may be omi ed in patients at risk for contrast-induced acute kidney injury, but the images produced are less distinct. When contrast is used, ensure that there is sufficient oral or IV intake to dilute and excrete the contrast media. Typically, the radiologist will specify a total fluid intake of 1 L or a variable rate to maintain urine output at 1 to 2 mL/kg/hr for up to 6 hours. When no contrast is used, there is no special postprocedural care. Contrast medium is potentially kidney damaging (nephrotoxic). Contrast-induced nephropathy is the onset of acute kidney failure within 48 hours after the administration of iodinated contrast medium (Lambert et al., 2017). The risk for contrast-induced nephropathy is greatest in patients who are older or dehydrated, have pre-existing chronic kidney disease (CKD), or have comorbidities of diabetes, heart failure, or current hypotension (Pagana & Pagana, 2018). Patients who take nephrotoxic drugs are also at risk. The best practice for patient safety and quality care lists assessment questions to ask before a patient undergoes testing with contrast material. In addition, patients taking metformin are at risk for lactic acidosis when they receive iodinated contrast media. Metformin should be discontinued at least 24 hours before the time of a procedure and for at least 48 hours after the procedure. Kidney function should be re-evaluated before the patient resumes metformin therapy. Nursing Safety Priority Drug Alert Ensure that the patient who is prescribed metformin does not receive the drug after a procedure requiring IV contrast material until adequate kidney function has been determined. Best Practice For Patient Safety & Quality Care Assessing the Patient About to Undergo a Kidney Test or Procedure Using Contrast Medium Before the procedure: • Ask the patient: • Have you had contrast medium before? If so, did you have a reaction? If so, describe the reaction (e.g., hives, facial edema, difficulty breathing, bronchospasm). If the patient has had a reaction before, he or she is at higher risk for having another reaction. • Do you have a history of asthma? Patients with asthma have been shown to be at greater risk for contrast reactions than the general public. When reactions do occur, they are more likely to be severe. • Do you have hay fever or food or drug allergies? Contrast reactions have been reported to be as high as 15% in patients with hay fever or food or drug allergies, especially to seafood, eggs, milk, or chocolate. • Are you taking metformin. Metformin must be discontinued at least 24 hours before any study using contrast media because the life-threatening complication of lactic acidosis, although rare, could occur. • When have you last eaten or drank anything? • Assess for a history of renal impairment and for conditions that have been implicated in increasing the chance of developing kidney injury or impairment after contrast media (e.g., diabetic nephropathy, class IV heart failure, dehydration, concomitant use of potentially nephrotoxic drugs such as the aminoglycosides or NSAIDs, and cirrhosis). • Assess hydration status by checking blood pressure, heart and respiratory rates, mucous membranes, skin turgor, and urine concentration. All patients at risk for contrast-induced nephrotoxicity need regular assessment and collaboration with the primary health care provider to maintain hydration and decrease the risk for kidney injury following a CT scan with IV contrast administration. IV fluids of normal saline are the most effective before the procedure to prevent contrast-induced nephrotoxic effects during radiologic procedures (Sethi et al., 2018). Diuretics may be given immediately after the contrast is injected to enhance excretion in patients who are well hydrated. Nursing Safety Priority Drug Alert When a CT scan with contrast is prescribed, report the patient’s history of immediate hypersensitivity reactions associated with the administration of contrast media to the radiologist and health care provider. Magnetic Resonance Imaging MRI provides improved imaging between normal and abnormal tissue in the renal system compared with a CT scan. As with all MRIs, the patient with metal implants (pins, pacemaker, joint replacement, aneurysmal clips, or other cosmetic or medical devices) is not eligible for this test because the magnet can move the metal implant, resulting in harm to the patient. A variation of MRI is magnetic resonance angiography (MRA). This noninvasive procedure is used to detect blockages in large arteries and can determine renal artery stenosis. Gadolinium-based contrast agents are used with MRI similar to CT scans. The contrast agent is injected intravenously and excreted via the kidneys. These agents have been linked with nephrogenic systemic fibrosis (Pagana & Pagana, 2018) and should not be used in patients with renal impairment, usually defined as a serum creatinine above 1.5 mg/dL (110 mcmol/L) or an estimated GFR less than 45 mL/min. Adults older than 60 years should be carefully evaluated for renal impairment. Kidney Ultrasonography Inform the patient that ultrasonography does not cause discomfort and is without risk. This test usually requires a full bladder. Ask the patient to drink 500 to 1000 mL of water, if needed, about 2 to 3 hours before the test to help fill the bladder. The patient should not void after drinking the water until the test is complete. This test applies sound waves to structures of different densities to produce images of the kidneys, ureters, and bladder and surrounding tissues. Ultrasonography allows assessment of kidney size, cortical thickness, and status of the calices. The test can identify obstruction in the urinary tract, tumors, cysts, and other masses without the use of contrast. In addition, it can determine blood flow into and out of the kidney using Doppler color flow imaging. The patient undergoing kidney ultrasound is usually placed in the prone position. Sonographic gel is applied to the skin over the back and flank areas to enhance sound wave conduction. A transducer in contact with and moving across the skin delivers sound waves and measures the echoes. Images of the internal structures are produced. Assisting the patient to a position of comfort and skin care to remove the gel is all that is needed after ultrasonography. Renal Scan This imaging test is used to examine the perfusion, function, and structure of the kidneys by the IV administration of a radioisotope. It does not use an iodinated contrast agent and thus may be used in preference to a CT scan when the patient is allergic to iodine or has impaired kidney function that places him or her at risk for kidney injury from IV contrast. No fasting or sedation is used. A peripheral IV catheter is inserted to give the radioisotope contrast agent. While the patient lies in a prone or si ing position, a camera is passed over the kidney area and records the isotope uptake on film, minutes after the radioisotope is given. After initial images, the patient may be given furosemide or captopril to be er visualize kidney function and blood flow. The isotope is eliminated 6 to 24 hours after the procedure. Encourage the patient to drink fluids to aid in excretion of the isotope. Because only tracer doses of radioisotopes are used, no precautions are needed related to radioactive exposure. Nursing Safety Priority Drug Alert g A renal scan is contraindicated in women who are pregnant unless the benefits outweigh the risks. Renal Arteriography (Angiography) Renal arteriography allows visualization of the renal arteries using a radiopaque contrast medium that enters the renal blood vessels and generates images to determine blood vessel size and abnormalities. The contrast medium is injected through the femoral or brachial artery as x-ray pictures are taken. This test has largely been replaced by other imaging techniques (e.g., nuclear renal scans, ultrasonography, computed tomography) and is seldom used as a stand-alone diagnostic procedure. The most common use of renal arteriography is at the time of a renal angioplasty or other intervention. Cystoscopy and Cystourethroscopy Patient Preparation Cystoscopy and cystourethroscopy are endoscopic procedures used to evaluate the bladder, urethra, and lower portions of the ureters. An endoscopy scope is inserted through the urethra into the bladder providing direct visualization. These procedures require completion of a preoperative checklist and a signed informed consent statement. The urologist provides a complete description of and reasons for the procedure, and the nurse reinforces this information. Cystoscopy may be performed for diagnosis or treatment. This test is used to examine for bladder trauma (cystoscopy) or urethral trauma (cystourethroscopy) and to identify causes of urinary tract obstruction. Cystoscopy also may be used to remove bladder tumors or plant radium seeds into a tumor, dilate the urethra and ureters with or without stent placement, stop areas of bleeding, or resect an enlarged prostate gland. Cystoscopy may be performed under general anesthesia or under local anesthesia with sedation. The patient’s age and general health and the expected duration of the procedure are considered in the decision about anesthesia. A light evening meal may be eaten. Usually the patient is NPO after midnight on the night before the cystoscopy. A bowel preparation with laxatives or enemas is performed the evening before the procedure so that bowel contents do not interfere with the procedure. Procedure The cystoscopy is performed in a designated cystoscopic examination room. If the procedure is performed in a surgical suite under general anesthesia, the usual surgical support personnel are present (see Chapter 9). This procedure is often performed in clinics, ambulatory surgery or short-procedure units, or a urologist’s office. Assist the patient onto a table and, after sedation, place the patient in the lithotomy position. After the anesthesia is given and the area cleansed and draped, the urologist inserts a cystoscope through the urethra into the urinary bladder. This examination commonly includes the use of both the cystoscope and urethroscope. Follow-up Care After this procedure with general anesthesia, the patient is returned to a postanesthesia care unit (PACU) or area. If local anesthesia and sedation were used, the patient may be returned directly to the hospital room. Patients undergoing cystoscopic examinations as outpatients are transferred to an area for monitoring before discharge to home. Monitor for airway patency and breathing, changes in vital signs (including temperature), and changes in urine output. Also observe for the complications of bladder puncture, excessive bleeding, and infection. Bladder puncture is accompanied by severe pain, including abdominal pain, nausea, and vomiting. A catheter may or may not be present after cystoscopy. The patient without a catheter has urinary frequency as a result of irritation from the procedure. The urine may be pink tinged, but gross bleeding is not expected. Bleeding or the presence of clots may obstruct the catheter and decrease urine output. Monitor urine output and notify the urologist of obvious blood clots or a decreased or absent urine output. Irrigate the Foley catheter with sterile saline, if prescribed. Notify the urologist if the patient has a fever (with or without chills) or an elevated white blood cell (WBC) count, which suggests infection. Urge the patient to take oral fluids to increase urine output (which helps prevent clo ing) and reduce the burning sensation on urination. Cystography and Cystourethrography These tests are a series of x-rays or a continuous radiographic visualization by fluoroscopy. During the imaging, radiopaque contrast medium fills the bladder and the bladder is emptied. Images show structure and function of the bladder and urethra. Tumors, rupture or perforation of the bladder and urethra, abnormal backflow of urine, and distortion from trauma or other pelvic masses can be seen. Patient Preparation and Procedure Explain the procedure to the patient. A urinary catheter is temporarily needed to instill contrast medium directly into the bladder for both procedures. The contrast medium enhances x-ray visibility of the lower urinary tract and is not absorbed into the bloodstream, reducing the risk for contrast-induced kidney injury. After bladder filling, x-rays are taken from the front, back, and side positions. For the voiding cystourethrogram (VCUG), the patient is requested to void and x-rays are taken during the voiding. A VCUG can determine whether urine refluxes (flow backward) into the ureter. The cystogram is used in cases of trauma when urethral or bladder injury is suspected or for patients with recurrent pyelonephritis (kidney infection). Follow-up Care Monitor for infection as a result of catheter placement. In this test, the contrast medium is not nephrotoxic because it does not enter the bloodstream and does not reach the kidney. Encourage fluid intake to dilute the urine and reduce the burning sensation from catheter irritation after removal. Monitor for changes in urine output because pelvic or urethral trauma may be present. Retrograde Procedures Retrograde means going against the normal flow of urine. A retrograde examination of the ureters and pelvis (pyelogram), the bladder (cystogram), and the urethra (urethrogram) involves instilling radiopaque contrast medium into the lower urinary tract. Because the contrast agent is instilled directly to obtain an outline of the structures desired, the agent does not enter the bloodstream. Therefore the patient is not at risk for contrastinduced kidney injury. The patient is prepared for retrograde procedures (retrograde pyelography, retrograde cystography, and retrograde urethrography) in the same way as for cystoscopy. Retrograde x-rays are obtained during the cystoscopy. After placement of the cystoscope by the urologist, catheters are placed into each ureter and contrast is instilled into each ureter and renal pelvis. The catheters are removed by the urologist, and x-rays are taken to outline these structures as the agent is excreted. The procedure identifies obstruction or structural abnormalities. For patients undergoing retrograde cystoscopy or urethrography, radiopaque contrast medium is instilled similarly into the bladder or urethra. Cystography and urethrography identify structural problems, such as fistulas, diverticula, and tumors. After retrograde procedures, monitor the patient for infection caused by placing instruments in the urinary tract. Because these procedures are performed during cystoscopic examination, follow-up care is the same as that for cystoscopy, including monitoring for bladder puncture or perforation. Other Diagnostic Assessments Urodynamic Studies Urodynamic studies examine the processes of voiding and include: • Tests of bladder capacity, pressure, and tone • Studies of urethral pressure and urine flow • Tests of perineal voluntary muscle function These tests are often used along with voiding urographic or cystoscopic procedures to evaluate problems with urine flow and disorders of the lower urinary tract. Cystometrography (CMG) can determine how well the bladder wall (detrusor) muscle functions and how sensitive it is to stretching as the bladder fills. This test provides information about bladder capacity, bladder pressure, and voiding reflexes. Explain the procedure and inform the patient that a urinary catheter will be needed temporarily during the procedure. Ask the patient to void normally. Record the amount and time of voiding. Insert a urinary catheter to measure the residual urine volume. The cystometer is a ached to the catheter, and fluid is instilled via the catheter into the bladder. The point at which the patient first notes a feeling of the urge to void and the point at which he or she notes a strong urge to void are recorded. Bladder capacity and bladder pressure readings are recorded graphically. The patient is asked to void when the bladder instillation is complete (about 500 mL). The residual urine after voiding is recorded, and the catheter is removed. Electromyography of the perineal muscles may be performed during this examination. For any procedure that involves inserting instruments into the urinary tract, monitor for infection. Record the patient’s temperature, the character of the urine, and urine output volume. Urethral pressure profile (also called a urethral pressure profilometry [UPP]) can provide information about the nature of urinary incontinence or urinary retention. Explain the procedure and inform the patient that a urinary catheter will be needed temporarily during the procedure. A special catheter with pressure-sensing capabilities is inserted into the bladder. Variations in the pressure of the smooth muscle of the urethra are recorded as the catheter is slowly withdrawn. As with any study involving inserting instruments into the urinary tract, monitor the patient for symptoms of infection. Urine stream testing is used to evaluate pelvic muscle strength and the effectiveness of pelvic muscles in stopping the flow of urine. It is useful in assessing urinary incontinence. Explain the procedure and reassure the patient that efforts will be made to ensure privacy. The patient is asked to begin urinating. Three to five seconds after urination begins, the examiner gives the patient a signal to stop urine flow. The length of time required to stop the flow of urine is recorded. Cleaning the perineal area, as after any voiding, is all that is necessary after the urine stream test. Electromyography (EMG) of the perineal muscles tests the strength of the muscles used in voiding. This information may help identify methods of improving continence. Inform the patient that some mild, temporary discomfort may accompany placement of the electrodes. In EMG of the perineal muscles, electrodes are placed in either the rectum or the urethra to measure muscle contraction and relaxation. After the completion of EMG, administer analgesics as prescribed to promote the patient’s comfort. NCLEX Examination Challenge 60.3 Safe and Effective Care Environment The nurse is admi ing a client undergoing a CT scan with contrast. Which finding does the nurse report as a possible immediate hypersensitivity reaction? Select all that apply. A. Nausea B. Pruritis C. Urticaria D. Laryngeal stridor E. Flushing of the skin Kidney Biopsy Patient Preparation Explain that a kidney biopsy can help determine a cause of unexplained kidney problems and help direct or change therapy. Most kidney biopsies are performed percutaneously (through skin and other tissues) using ultrasound or CT guidance. The patient signs an informed consent. Patients are NPO for 4 to 6 hours before the procedure. Because of the risk for bleeding after the biopsy, coagulation studies such as platelet count, activated partial thromboplastin time (aPTT), prothrombin time (PT), and bleeding time are performed before surgery. Hypertension is aggressively managed before and after the procedure because high blood pressure can make stopping the bleeding after the biopsy more difficult. Uremia also increases the risk for bleeding, and dialysis may be prescribed before a biopsy. A blood transfusion may be needed to correct anemia before biopsy. Procedure In a percutaneous biopsy, the nephrologist or radiologist obtains tissue samples without an incision. Patients receive sedation and are monitored throughout the procedure. The patient is placed in the prone position on the procedure table. The entry site is selected after taking preliminary images. The area is prepped and sterilely draped. A local anesthetic is injected, and the physician then inserts the biopsy device into the tissues toward the kidney. Needle depth and placement are confirmed by ultrasound or CT. While the patient holds his or her breath, the needle is advanced into the renal cortex. Samples are then taken with a springloaded coring biopsy needle and sent for pathologic study (Pagana & Pagana, 2018). Follow-up Care After a percutaneous biopsy, the major risk is bleeding into the kidney or the tissues external from the kidney at the biopsy site. For 24 hours after the biopsy, monitor the dressing site, vital signs (especially fluctuations in blood pressure), urine output, hemoglobin level, and hematocrit. Even if the dressing is dry and there is no hematoma, the patient could be bleeding from the site. An internal bleed is not readily visible but is suspected with flank pain, decreasing blood pressure, decreasing urine output, or other signs of hypovolemia or shock. With severe bleeding, some patients develop bruising along the flank and back accompanied by pain. The patient follows a plan of strict bedrest, lying in a supine position with a back roll for additional support for 2 to 6 hours after the biopsy. The head of the bed may be elevated, and the patient may resume oral intake of food and fluids. After bedrest, the patient may have limited bathroom privileges if there is no evidence of bleeding. Monitor for hematuria, the most common complication of kidney biopsy. Hematuria occurs microscopically in most patients, but 5% to 9% have gross hematuria. This problem usually resolves without treatment 48 to 72 hours after the biopsy but can persist for 2 to 3 weeks. In rare cases, transfusions and surgery are required. There should be no obvious blood clots in the urine. The patient may have some local pain after the biopsy. If aching originates at the biopsy site and begins to radiate to the flank, back, and around the front of the abdomen, bleeding may have started, or a hematoma is forming around the kidney. This pa ern of pain with bleeding occurs because blood in the tissues around the kidney increases pressure on local nerve tracts. If bleeding occurs, IV fluid, packed red blood cells, or both may be needed to prevent shock. In general, a small amount of bleeding creates enough pressure to compress bleeding sites. This is called a tamponade effect. If tamponade does not occur and bleeding is extensive, surgery for hemostasis or even nephrectomy may be needed. A hematoma in, on, or around the kidney may become infected, requiring treatment with antibiotics and surgical drainage. If no bleeding occurs, the patient can resume general activities after 24 hours. Instruct the patient to avoid lifting heavy objects, exercising, or performing other strenuous activities for 1 to 2 weeks after the biopsy procedure. Driving may also be restricted. Refer to Chapter 9 for general postoperative care for the patient who has undergone an open kidney biopsy. NCLEX Examination Challenge 60.4 Safe and Effective Care Environment Which assessment finding would require the nurse to take immediate action in a client who is 1 hour post kidney biopsy? Select all that apply. A. Pink-tinged urine B. Nausea and vomiting C. Increased bowel sounds D. Reports of flank pain E. The patient is ambulating to the bathroom Clinical Judgment Challenge 60.1 Safety; Patient-Centered Care The nurse is assessing a 42-year-old female client who is scheduled for surgical repair of a hip fracture from a car crash 4 hours ago. The client was traveling approximately 40 miles per hour when she struck a guard rail after a deer ran into the roadway. The client was wearing a seat belt at the time of the accident. The client reports pain in the left hip area with noted swelling. There is a 2-cm abrasion over the right eye and a contusion on the left upper forearm. When the client voids, the nurse assesses that the urine is rust colored and the client states “It burns when I urinate.” The client has a history of anxiety. 1. Recognize Cues: What assessment information in this client situation is the most important and immediate concern for the nurse? (Hint: Identify the relevant information first to determine what is most important.) 2. Analyze Cues: What client conditions are consistent with the most relevant information? (Hint: Think about priority collaborative problems that support and contradict the information presented in this situation.) Get Ready for the Next-Generation NCLEX® Examination! Key Points Review these Key Points for each NCLEX Examination Client Needs Category. Safe and Effective Care Environment • Wear gloves when handling urine or drainage from the genitourinary tract. QSEN: Safety • Evaluate the patient for potential adverse or allergic reactions to radiopaque contrast agents, iodine, or gadolinium. QSEN: Safety • Assess the patient for use of drugs that increase risk for kidney dysfunction. QSEN: Safety • Assess the patient for bleeding, increased pain, and symptoms of perforation or infection after any invasive test of kidney/urinary function. QSEN: Safety • Inform primary health care providers about any symptoms of complications following invasive or noninvasive tests of urinary and kidney structure or function. QSEN: Safety Health Promotion and Maintenance • Teach patients to clean the perineal area after voiding, after having a bowel movement, and after sexual intercourse. QSEN: Evidence-Based Practice • Urge all patients to maintain an adequate fluid intake (sufficient to dilute urine to a light yellow color) unless another health problem requires fluid restriction. QSEN: Evidence-Based Practice Psychosocial Integrity • Allow the patient to express fear or anxiety about renal system diagnostic tests and renal system alterations. QSEN: Patient-Centered Care • Provide privacy for patients undergoing examination or testing of the renal system. QSEN: Patient-Centered Care • Use language and terminology that the patient can understand during discussions of kidney/urinary assessment. QSEN: Patient-Centered Care Physiological Integrity • Ask the patient about kidney problems in any other family members because some problems have a genetic component. QSEN: PatientCentered Care • Explain all diagnostic procedures, restrictions, and follow-up care to the patient scheduled for tests. QSEN: Patient-Centered Care • Interpret laboratory data to distinguish between dehydration and kidney impairment. QSEN: Evidence-Based Practice • Describe how to obtain different types of urine specimens. QSEN: Evidence-Based Practice • Document renal and urinary system assessment in the patient’s electronic health record. QSEN: Patient-Centered Care • Assess urine and serum tests of kidney function closely after renal system diagnostic tests. QSEN: Evidence-Based Practice Mastery Questions 1. Which client being managed for dehydration does the nurse consider at greatest risk for possible reduced kidney function? A. An 80-year-old man who has benign prostatic hyperplasia B. A 62-year-old woman with a known allergy to contrast media C. A 48-year-old woman with established urinary incontinence D. A 45-year-old man receiving oral and intravenous fluid therapy 2. Which client assessment data is essential for the nurse to report to the health care provider before a renal scan is performed? A. Pink-tinged urine B. Reports pregnancy C. Reports claustrophobia D. History of an aneurysm clip 3. Which lab finding is indicative of renal function alterations and not dehydration? Select all that apply. A. BUN 20 mL/dL B. Creatinine 2.3 mL/dL C. Hemoglobin 14 g/dL D. Cystatin-c 105 mg/mL E. BUN/creatinine ratio 10 F. Creatinine clearance 175 mL/min 4. Which symptom(s) in a client during the first 12 hours after a kidney biopsy indicates to the nurse a possible complication from the procedure? A. The client experiences nausea and vomiting after drinking juice. B. The biopsy site is tender to light palpation. C. The abdomen is distended, and the client reports abdominal discomfort. D. The heart rate is 118, blood pressure is 108/50, and peripheral pulses are thready. References Brenner B.M, ed. Brenner & Rector’s the kidney . 10th ed. Philadelphia: Saunders; 2016. Burchum J, Rosenthal L. Lehne’s pharmacology for nursing care . 10th ed. St. Louis: Elsevier; 2019. Denic A, Glassock R, Rule A. Structural and functional changes with the aging kidney . 2016;23(1) doi: 10.1053/j.ackd.2015.08.004. Jackson C, Botelho E, Josepf J, Tennstedt S. Accessing and evaluating urologic health information: Differences by race/ethnicity and gender. Urologic Nursing . 2013;33(6):282–287. Jarvis C. Physical examination & health assessment . 8th ed. St. Louis: Elsevier; 2020. Lambert P, Chasson K, Horton S, Petrin C, Marshall E, Bowdon S, et al. Reducing acute kidney injury due to contrast material: How nurses can improve patient safety. Critical Care Nurse . 2017;37(1):13–26.