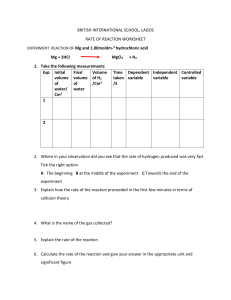
Head to savemyexams.com for more awesome resources 12.1 Experimental Techniques Question Paper Course CIE IGCSE Chemistry Section 12. Experimental Techniques & Chemical Analysis Topic 12.1 Experimental Techniques Difficulty Easy Time allowed: 40 Score: /27 Percentage: /100 Page 1 of 8 © 2015-2023 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Head to savemyexams.com for more awesome resources Question 1a A student does three titrations with dilute hydrochloric acid and potassium hydroxide solution. Complete the equation to show the products formed during this reaction. potassium hydroxide + hydrochloric acid → potassium ______ + _______ [2 marks] Question 1b Some of the apparatus the student uses is shown below. Name the piece of equipment the student will use to measure out the 25.0 cm3 of potassium hydroxide solution. [1 mark] Question 1c In her first titration the student measures the initial volume of hydrochloric acid in the burette. She slowly adds the acid until the potassium hydroxide is just neutralised. She then measures the volume of the hydrochloric acid again. Describe how she can tell when the potassium hydroxide solution is just neutralised. Page 2 of 8 © 2015-2023 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Head to savemyexams.com for more awesome resources [1 mark] Question 1d Look at the diagrams. They show parts of the burette during the first titration. Here is the student’s results table. Titration number final reading in cm3 initial reading in cm3 1 titre (volume of acid added) in cm3 2 37.5 20.4 3 32.1 15.0 17.1 17.1 Complete the table by recording the burette readings from the diagrams. [2 marks] Page 3 of 8 © 2015-2023 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Head to savemyexams.com for more awesome resources Question 2a The names of some pieces of equipment are shown. stopwatch balance thermometer gas syringe burette pipette For each question, name the piece of apparatus that would be used in each case. Each piece of equipment can be used once, more than once or not at all. Name the piece of apparatus that would be used to measure the time taken for a white precipitate to form when barium chloride is added to sodium sulfate. [1 mark] Question 2b Name the piece of apparatus that would be used to determine whether the reaction between magnesium and hydrochloric acid is exothermic. [1 mark] Question 2c Name the piece of apparatus that would be used to measure the volume of carbon dioxide produced when marble chips are added to hydrochloric acid. [1 mark] Question 2d Name the piece of apparatus used to add hydrochloric acid to 25 cm3 of sodium hydroxide solution during a titration. [1 mark] Question 3a A student investigated the effect of temperature on the mass of sodium chloride that dissolves in 100 cm3 of water. Name the piece of apparatus that would be used to measure the mass of sodium chloride. [1 mark] Page 4 of 8 © 2015-2023 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Head to savemyexams.com for more awesome resources Question 3b A control variable is one which must be kept constant each time the investigation is repeated. Give one control variable in this investigation. The temperature of the water The volume of water The mass of sodium chloride The beaker used [1 mark] Question 3c Complete these sentences using the word from the list. solution residue saturated solute filtrate solvent liquid The salt is the .................................. because it dissolves in water. The water is the .................................. because it has the salt dissolved in it. The mixture of salt and water is known as a ................................... When no more salt can dissolve in water at a specific temperature, the solution has become.................................. [4 marks] Page 5 of 8 © 2015-2023 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Head to savemyexams.com for more awesome resources Question 3d Separate: Chemistry Only The student used a thermometer to measure the temperature during their investigation. Name a different piece of apparatus that would give the student a more precise reading. [1 mark] Question 4a A student made copper sulfate crystals. The first two steps of their method are shown below. Why should sulfuric acid be added in excess to copper carbonate? [1 mark] Question 4b The contents of the beaker in step 2 are separated using filtration. Identify the residue. Draw a circle around the correct answer. water copper carbonate copper(II) sulfate Page 6 of 8 © 2015-2023 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers sulfuric acid Head to savemyexams.com for more awesome resources [1 mark] Question 4c Identify the filtrate. Draw a circle around the correct answer. water copper carbonate copper(II) sulfate sulfuric acid [1 mark] Question 4d Describe how you would obtain dry copper(II) sulfate crystals from the solution. [2 marks] Question 5a A student carried out a titration using sodium hydroxide and hydrochloric acid. Their method is written below. A B C D E Add a few drops of phenolphthalein Add the HCl slowly until the endpoint is reached Add HCl into the burette and record initial volume Pipette 25 cm3 of NaOH into a conical flask Record the volume of acid added Put the statements A, B, C, D, E in the correct order. The first step has been done for you. first step C last step Page 7 of 8 © 2015-2023 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers Head to savemyexams.com for more awesome resources [2 marks] Question 5b What colour change will be observed when the end point is reached? Tick one box. Red to yellow Pink to colourless Colourless to pink Yellow to red [1 mark] Question 5c Why can universal indicator not be used to show the end point in a titration? [1 mark] Question 5d Separate: Chemistry Only A measuring cylinder could have been used to measure 25 cm3 of sodium hydroxide. Give one advantage of using a pipette [1 mark] Page 8 of 8 © 2015-2023 Save My Exams, Ltd. · Revision Notes, Topic Questions, Past Papers