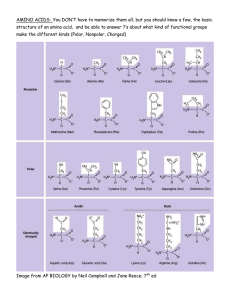
1 Practices – Chapters 1-4 1. Which biomolecule is the most abundant to nitrogen element? A. carbohydrates B. lipids C. nucleic acids D. proteins 2. What is the name of the functional group present in the following molecule? R-O-R A. amide B. carbonyl C. ether D. ester 3. A glycosidic bond is what chemical functional group? A. amide B. phosphoester C. ether D. hydroxyl 4. Which of the following is a monosaccharide? A. C2H4O2 B. C2H5OH C. C3H6O3 D. C2H5NO 5. Which of the following biomolecules are known primarily for their low solubility in water? A. carbohydrates B. lipids C. nucleic acids D. proteins 6. Calculate the free energy change, ∆G, for the following reaction at 25 °C: CO(g) + 2 H2(g) CH3OH(l) ∆H for this reaction is -127.9 kJ/mol, and ∆S for this reaction = -0.3319 kJ/(K • mol). A. –150.8 kJ/mol B. –86.8 kJ/mol C. –29.0 kJ/mol D. +150.8 kJ/mol 7. A particular reaction has ∆G = -55.6 kJ/mol. This reaction is A. spontaneous B. not spontaneous C. at equilibrium 8. Consider the following reaction: glucose-1-phosphate → glucose + Pi ∆G = -20.9 kJ With which reaction below could this reaction be coupled to yield a non-spontaneous reaction? 2 A. glycerol + Pi glycerol-3-phosphate B. glycerol-3-phosphate glycerol + Pi C. glucose-6-phosphate glucose + Pi D. ADP + Pi ATP ∆G = +9.2 kJ ∆G = –9.2 kJ ∆G = –13.8 kJ ∆G = +30.5 kJ 9. One of the steps of the citric acid cycle is the following reaction: In this reaction A. malate is reduced comparing to oxaloacetate B. malate is oxidized comparing to oxaloacetate 10. Which of the following have cells that do not contain a nucleus? A. archaea B. bacteri C. eukaryotes D. prokaryotes 11. The electron-pair geometry of water is a , and the molecular shape of water is A. a = tetrahedral; b = bent B. a = tetrahedral; b = trigonal pyramidal C. a= trigonal planar; b = bent D. a = trigonal planar; b = trigonal pyramidal b . 3 12. Carbonyl group, whose structure is shown below, is a polar molecule. What intermolecular forces exist between molecules of with the carbonyl group? A. London dispersion forces B. dipole-dipole forces C. London dispersion forces and dipole-dipole forces D. London dispersion forces, dipole-dipole forces, and hydrogen bonds 13. Polar molecules tend to _ a . This phenomenon is referred to as the b A. a = dissolve in polar solvents; b = hydrophilic B. a = aggregate in polar solvents; b = hydrophobic C. a = get as far apart as possible in polar solvents; b = hydrophilic D. a = get as far apart as possible in polar solvents; b = hydrophobic 14. Which of the following cannot form a bilayer? A. hydrophilic materials C. hydrophobic materials 15. What is the pH of an aqueous solution that has [H+] = 5.7 × 10–3 M? A. 11.83 B. 6.38 C. 3.00 D. 2.24 16. A basic solution has a pH that is A. equal to 7 B. greater than 7 C. less than 7 17. In the reaction for the ionization of acetic acid (CH3COOH) in water CH3CH2COOH + H2O <=> H3O+ + CH3CH2COO– the conjugate base of acetic acid is A. CH3CH2COO– B. H2O C. H3O+ 18. Which of the following acids is weaker? A. acetic acid, pKa = 4.7 B. benzoic acid, pKa = 4.2 C. the side chain of aspartic acid, pKa = 3.9 D. the side chain of glutamic acid, pKa = 4.1 effect. 4 19. Consider the following reaction between the amino acids histidine and serine. In this reaction, histidine is acting as a(n) A. a = acid; b = acid B. a = acid; b = base C. a = base; b = acid D. a = base; b = base a , and serine is acting as a(n) b . 20. The structure of one of the nitrogenous bases found in nucleic acids is shown below: This is the structure of A. adenine B. cytosine C. uracil D. thymine 5 21. Given that the pKa of the carboxylic acid group in an amino acid is about 4 and that of the protonated amino group in an amino acid is about 10, predict the species that will be present in an aqueous solution with a pH of 3. A. B. C. D. 22. The following molecule is A. deoxyglucose B. deoxyribose C. glucose D. ribose 23. How many hydrogen bonds are present in a base pair between G and C? A. 1 B. 2 C. 3 D. 4 24. What is the name of the following molecule? 6 A. adenosine 5’-monophosphate B. guanine 3’-monophosphate C. guanine 5’-monophosphate D. guanosine 5’-diphosphate 25. Which vitamin is needed for the synthesis of coenzyme A? A. niacin B. B5 C. riboflavin D. tocopherol 26. Which vitamin is needed for the synthesis of NAD? A. B3 B. pantothenic acid C. riboflavin D. tocopherol 27. In double stranded DNA, the base pairs are located groups are located b of the double helix. A. a = on the exterior; b = on the exterior B. a = on the exterior; b = in the interior C. a = in the interior; b = on the exterior D. a = in the interior; b = in the interior a of the double helix, and the phosphate 28. Rapid cooling to temperature lower than melting temperature, DNA will be present as A. double stranded molecules B. single stranded molecules C. double stranded molecules with improper base pairing 29. The process of making proteins from an RNA template is called A. replication B. transcription C. translation 7 30. Given the following sequence of mRNA in an open reading frame, what polypeptide sequence would be produced? 5’-GAUUGGCUU-3’ A. Asp-Phe-Gly-Leu B. Asp-Trp-Leu C. Leu-Trp-Asp D. Phe-Gly-Stop 31. What is the name of the following amino acid? A. asparagine B. aspartate C. glutamine D. glycine 32. What is the three letter code for the following amino acid? 8 A. Phe B. Thr C. Trp D. Tyr 33. The structure of the amino acid aspartate is shown below: The sidechain of aspartate is classified as A. nonpolar B. polar, uncharged C. polar, charged 34. Amino acids with side chains are most likely to be found in the exterior of a globular protein. A. nonpolar B. polar, charged 35. This level of protein structure is the spatial arrangement of the polypeptide backbone. A. primary B. secondary C. tertiary D. quaternary 36. This level of protein structure is the three-dimensional structure of an entire polypeptide, including all its side chains. A. primary B. secondary C. tertiary D. quaternary 37. What is the net charge of the following tetrapeptide at a pH of 12: Asp-Arg-Ala? 9 A. –2 B. –1 C. 0 D. +1 38. In an α-helix, a carbonyl is hydrogen bonded to an amino hydrogen A. 15 B. 10 C. 5 D. 4 atoms away. 39. What is the name for a region that has a local three-dimensional structure in a single polypeptide chain? A. domain B. ordinate C. range D. subunit 10 40. What type of secondary structure is illustrated in the following figure? A. α-helix B. antiparallel β-sheet C. parallel β-sheet