
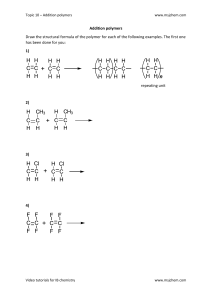
²⁴th February 2023 TITLE 1. HEADING 1 To get started right away, just tap any placeholder text (such as this) and start typing. A. Heading 2 i. POLYMERS Polymers are materials of high molecular weight that have many applications in modern society. They usually consist of several structural units bound together by covalent bonds. For example, polyethylene (PE) is a long -chain polymer & is represented as -CH²-CH²-CH² or [-CH²CH2-]n Where the structural unit is -CH2-CH2- and n represents the chain length of the polymer. Polymers are obtained through the combination of small molecules called monomers. For example, polyethylene is formed from the monomer ethylene. In order to form polymers, monomers should either have reactive functional groups or a double bond whose reaction provides the necessary linkages between the repeat units. Polymeric materials find extensive use because of their high strength, & other unique properties such as light weight, high strength to -weight ratio, processing advantages, corrosion resistance, etc. They are extensively used in food packaging, clothing, home furnishing, transportation, medical devices, information technology, etc. Natural fibres such as silk, wool & cotton are polymers & have been used for thousands of years. Synthetic polymers such as polyolefins, polyesters, acrylics, nylons, etc. In the past few years have replaced them extensively as clothing & protective materials. It may be added that biological materials such as proteins, deoxyribonucleic acid (DNA) & polysaccharides are also polymers. Many synthetic polymeric materials, known as biomaterials, are increasingly being employed within the human body either as artificial organs, bone cements, dental cements, ligaments, pacemakers or contact lenses because they are inert & hence, not rejected by the body. These include natural polymeric materials, which are derived from animals or plants such as cellulose, chitin, dextran, agarose & collagen, as well as polymeric materials such as polysiloxane , polyurethane, polymethyl, methacrylate, polyacrylamide, polyester & polyethylene oxides. It’s predicted that within a few years, polymers may replace more conventional materials for various applications. As a result, a large percentage of chemists & engineers are engaged in the work involving polymers, which necessitates the study of polymer science as a separate subject. This is also needed because their behaviours as materials is different from that of metals & other materials having low molecular weight. SOME IMPORTANT TERMS & DEFINITIONS 1. Monomers. These are simple molecules , which combines with each other to form polymers. They are also called building blocks of polymers. For example, ethylene, methylmethacrylate , polyvinyl chloride, etc. 2. Polymer: It’s a macromolecule formed by the repeated unit of several simple molecules called monomers. The process of linking the repeating units is called polymerization. 3. POLYMERIZATION: IT’S THE PROCESS OF CONVERSION OF SUBSTANCES HAVING LOW MOLECULAR WEIGHT INTO SUBSTANCES HAVING HIGH MOLECULAR WEIGHT WITH OR WITHOUT THE ELIMINATION OF BYPRODUCTS SUCH AS HCL, H2O, NH³, ETC.POLYMERISATION REACTION USUALLY TAKES PLACE IN THE PRESENCE OF INITIATORS. 4. DEGREE OF POLYMERIZATION: IT’S THE NO NUMBER OF REPEATING UNITS PRESENT IN A POLYMER. 5. FUNCTIONALITY: IT’S THE TOTAL NUMBER OF BONDING SITES OR FUNCTIONAL GROUPS PRESENT IN A MONOMER MOLECULE. 2

![\t<L Your Name: _[printed]](http://s2.studylib.net/store/data/013223479_1-5f2dc062f9b1decaffac7397375b3984-300x300.png)