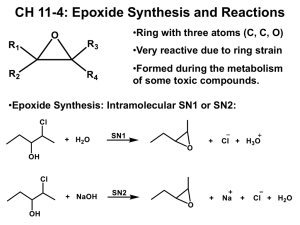
O 1) NaCN 2) H2O H ? O O H H This chirality center will be inverted 2˚ (Less hindered) 3˚ @ O @ O C H H C H HO O N C Proton transfer N N Nucleophilic attack Q For each of the following reactions, predict the product and draw a mechanism for its formation: O (a) Me O (c) Me 1) PhMgBr 2) H2O 1) NaSH 2) H2O O (e) Et Me ? ? 1) NaSH 2) H2O O (b) 1) NaCN 2) H2O Me O (d) ? 1) LAH 2) H2O Me O (f) Et H H ? ? 1) LAH Me 2) H2O ? Predict the product of the reaction below and draw the likely mechanism for its formation: O Et Me ? [H2SO4] Et EtOH H Nu attack where stability of C+ is more Back side attack Proton transfer H H O Et H Me 14.20 ! SN 2 ! O Et Et Et Me Et H SN1 having character Et O H OH Me Et Et O Et ! H H For each reaction, predict the product and draw a mechanism for its formation: O HCl (a) ? O Me Et (c) O (e) H O Me O (b) [H2SO4] EtOH [H2SO4] MeOH ? ? Me O Me (d) ? HBr H2 O Me Et ? [H2SO4] O HBr (f) (a) Under acidic conditions, the epoxide is protonated, thereby generating a very powerful electrophile (a protonated epoxide). Since the starting epoxide is symmetrical, regiochemistry is not a concern in this case. That is, the nucleophile can attack the epoxide at either position, giving the same product either way. Stereochemistry is also not an issue in this case, because the product does not contain any chiral centers. Et Me ? competition is between a secondary position and a tertiary position, electronic factors dominate and the tertiary position is attacked. Back-side attack causes inversion of configuration at the chiral center being attacked. Finally, a proton is removed (the most likely base is the solvent, ethanol). protonated epoxide). Methanol (CH3OH) is a weak nucleophile, and we must decide which position will be attacked. In this case, one position (left) is tertiary, and the other position (right) is secondary. When the competition is between a secondary position and a tertiary position, electronic factors dominate and the tertiary position is attacked. Back-side attack causes inversion of configuration at the chiral center being attacked. Finally, a proton is removed (the most likely base is the solvent, methanol). O OH [H2SO4] H Me MeOH H O H H Me MeO H MeOH Me O (b) Under acidic conditions, the epoxide is protonated, thereby generating a very powerful electrophile (a protonated epoxide). To determine where the nucleophile (bromide) attacks, we must decide whether steric or electronic effects dominate. In this case, one position (left) is primary and the other position (right) is secondary. Under these conditions, steric effects will dominate, and the attack is expected to occur at the less substituted position. The position being attacked is not a chiral center. There is an existing chiral center, but that center is not attacked, so we do not expect the configuration of that chiral center to change. (d) Under acidic conditions, the epoxide is protonated, thereby generating a very powerful electrophile (a protonated epoxide). Water (H2O) is a weak nucleophile, and we must decide which position will be attacked. In this case, one position (left) is tertiary, and the other position (right) is secondary. When the competition is between a secondary position and a tertiary position, electronic factors dominate and the tertiary position is attacked. Back-side attack causes inversion of configuration at the chiral center being attacked. Finally, a proton is removed (the most likely base is the solvent, water). OH H Me MeOH Me H (f) Under acidic conditions, the epoxide is protonated, thereby generating a very powerful electrophile (a protonated epoxide). Bromide is a nucleophile, and we must decide which position will be attacked. In this case, one position (left) is tertiary, and the other position (right) is secondary. When the competition is between a secondary position and a tertiary position, electronic factors dominate and the tertiary position is attacked. Back-side attack causes inversion of configuration at the chiral center being attacked. O Et H Me Br Et HBr Br H H Br O Et (e) Under acidic conditions, the epoxide is protonated, thereby generating a very powerful electrophile (a (c) Under acidic conditions, the epoxide is protonated, thereby generating a very powerful electrophile (a protonated epoxide). Ethanol (EtOH) is a weak nucleophile, and we must decide which position will be attacked. In this case, one position (left) is tertiary, and the other position (right) is secondary. When the H Me O H Me OH H Me