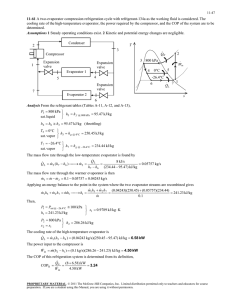
Evaporation Introduction The objective of evaporation is to concentrate a solution consisting of a nonvolatile solute and a volatile solvent. In the overwhelming majority of evaporations the solvent is water. When the liquid phase is agitated, mass-transfer in the liquid phase is sufficiently rapid that the rate of evaporation of solvent can be determined by the rate of heat transfer from the heating medium, usually condensing steam, to the solution. Evaporation differs from drying in that the residue is a liquid-sometimes a highly viscous one-rather than a solid. it differs from distillation in that the vapor usually is a single component, and even when the vapor is a mixture, no attempt is made in the evaporation step to separate the vapor into fractions. Mineral-bearing water often is evaporated to give a solid-free product for boiler feed, for special process requirements, or for human consumption. This technique is often called water distillation, but technically it is evaporation. Liquid characteristics 1. Concentration •The density and viscosity increase with solid content until either the solution becomes saturated or the liquor becomes too viscous for adequate heat transfer. •continued boiling of a saturated solution causes crystals to form; these must be removed or the tubes clog. •The boiling point of the solution may also rise considerably as the solid content increases, so that the boiling temperature of a concentrated solution may be much higher than that of water at the same pressure. 2. Foaming A stable foam accompanies the vapor out of the evaporator, causing heavy entrainment. In extreme cases the entire mass of liquid may boil over into the vapor outlet and be lost. 3. Temperature sensitivity 4. Scale رسوب 5. Materials of construction 6. Toxicity, explosion hazards, radioactivity, and necessity for sterile operation Once-through circulation evaporators These evaporators are well adapted to multiple-effect operation Agitated-film evaporators are always operated once through Falling-film and climbing-film evaporators can also be operated in this way Useful for heat-sensitive materials, By operating under high vacuum, the temperature of the liquid can be kept low With a single rapid passage through the tubes the thick liquor is at the evaporation Temperature but a short time and can be quickly cooled as soon as it leaves the evaporator Circulation evaporators Although the average residence time of the liquid in the heating zone may be short, part of the liquid is retained in the evaporator for a considerable time. Prolonged heating of even a small part of a heat-sensitive material like a food can ruin the entire product. Climbing-film evaporators are usually circulation units. Continuous flow evaporators 1. Horizontal-tube evaporator. inside of which steam condenses and outside of which the solution to be concentrated boils. Agitation is provided only by the movement of the bubbles formed. Therefore, this type of unit is only suitable for low-viscosity solutions that do not deposit scale on the heat-transfer surfaces. Continuous flow evaporators 2. Short-vertical-tube evaporator. solution inside the tubes and condensing outside. steam Boiling inside the tube causes the solution to circulate, thus providing additional agitation not suitable for very viscous solutions. Continuous flow evaporators 3. Long-vertical-tube evaporator Higher tube-entering liquid velocity Higher heat-transfer coefficient For liquids that tend to foam. Continuous flow evaporators 4. Forced-circulation evaporator Very viscous solutions A pump is used to force the solution upward Through relatively short tubes Because of the high velocities in a forcedcirculation evaporator, the residence time of the liquid in the tubes is short-about 1 to 3 sso that moderately heat-sensitive liquids can be concentrated in them. Salting liquors or those that tend to foam. Continuous flow evaporators 5. Falling-film evaporator heat-sensitive solutions such as fruit juices The flows as a film inside walls of the tubes The concentrate and the vapor produced are separated at the bottom Continuous flow evaporators 6. AGITATED-FILM EVAPORATOR The principal resistance to overall heat transfer from the steam to the boiling liquid in an evaporator is on the liquid side. One way of reducing this resistance, especially with viscous liquids, is by mechanical agitation of the liquid film. This is a modified falling-film evaporator with a single jacketed tube containing an internal agitator. High rates of heat transfer with viscous liquids Viscous heat-sensitive products as gelatin, rubber latex, antibiotics, and fruit juices. Performance of tubular evaporators Capacity is defined as the number of kilograms of water vaporized per hour. Economy is the number of kilograms vaporized per kilogram of steam fed to the unit. In a single-effect evaporator the economy is nearly always less than 1, but in multiple-effect equipment it may be considerably greater. The steam consumption, in kilograms per hour, is also important. It equals the capacity divided by the economy. The rate of heat transfer q through the heating surface of an evaporator is the product of three factors: the area of the heat-transfer surface A, the overall heattransfer coefficient U, and the overall temperature drop ΔT. q = UAΔT Evaporator Economy Influencing factor 1. Number of effects By proper design the enthalpy of vaporization of the steam to the first effect can be used one or more times, depending on the number of effects. 2. Temperature of the feed Effect of the feed state on the capacity If the feed to the evaporator is at the boiling temperature corresponding to the absolute pressure in the vapor space, all the heat transferred through the heating surface is available for evaporation and the capacity is proportional to q. If the feed is cold, the heat required to heat it to its boiling point may be quite large and the capacity for a given value of q is reduced accordingly, as heat used to heat the feed is not available for evaporation. if the feed is at a temperature above the boiling point in the vapor space, a portion of the feed evaporates spontaneously by adiabatic equilibration with the vapor-space pressure and the capacity is greater than that corresponding to q. This process is called flash evaporation. Boiling-point elevation (BPE) For a given pressure in the vapor space of an evaporator, the boiling temperature of an aqueous solution will be equal to that of pure water if the solute is not dissolved in the water but rather consists of small, insoluble, colloidal material. If the solute is soluble, the boiling temperature will be greater than that of pure water by an amount known as the boiling-point elevation of the solution. In actual evaporators, however, the boiling point of a solution is affected by two factors, boiling-point elevation and liquid head. If, as is usually the case, the solute has little or no vapor pressure, the evaporator pressure is equal to the partial pressure of the water in the solution. Then, by a modified Raoult's law: Diihring chart for aqueous solutions of sodium hydroxide. Nomograph for boiling-point elevation of aqueous solutions Effect of liquid head and friction on temperature drop If the depth of liquid in an evaporator is appreciable, the boiling point corresponding to the pressure in the vapor space is the boiling point of the surface layer of liquid only. The average boiling point of the liquid in the tubes is higher than the boiling point corresponding to the pressure in the vapor because: 1. pressure of the vapor space 2. head of Z meters or feet of liquid 3. frictional loss in the tubes increases the average pressure of the liquid (large liquid velocity). This increase in boiling point lowers the average temperature drop between the steam and the liquid and reduces the capacity. The amount of reduction cannot be estimated quantitatively with precision, but the qualitative effect of liquid head, especially with high liquor levels and high liquid velocities, should not be ignored. The temperature drop is fixed by the properties of the steam and the boiling liquid and except for the effect of hydrostatic head is not a function of the evaporator construction. Heat transfer coefficients The overall coefficient, on the other hand, is strongly influenced by the design and method of operation of the evaporator. the overall resistance to heat transfer between the steam and the boiling liquid is the sum of five individual resistances: 1. The steam-film resistance (not important, the presence of non-condensable gas seriously reduces the steam-film coefficient) 2. Inside and outside the tubes 3. The tube-wall resistance (not important) 4. The resistance from the boiling liquid. The liquid-side coefficient The liquid-side coefficient depends to a large extent on the velocity of the liquid over the heated surface. In most evaporators, and especially those handling viscous materials, the resistance of the liquid side controls the overall rate of heat transfer to the boiling liquid. Forced circulation gives high liquid-side coefficients even though boiling inside the tubes is suppressed by the high static head. The formation of scale on the tubes of an evaporator adds a thermal resistance equivalent to a fouling factor. single-effect evaporation When a single evaporator is used, the vapor from the boiling liquid is condensed and discarded. This method is called single-effect evaporation, and although it is simple, it utilizes steam ineffectively. To evaporate 1 kg of water from a solution calls for from 1 to 1.3 kg of steam. Continuous-flow, steady-state model evaporator 1. The thin-liquor feed has only one volatile component, e.g., water. 2. Only the latent heat of the heating steam at T, is available for heating and vaporizing the solution in the evaporator. 3. The boiling action on the heat-exchanger surfaces agitates the solution, in the evaporator, sufficiently to achieve perfect mixing Te= Tp and Tv = Tp. 4. Driving force for heat transfer = ΔT = Ts Tp 5. The ΔT is high enough to achieve nucleate boiling and not so high as to cause film boiling 6. No heat loss from the evaporator weight-fraction solute: wf mass flow rate: mf Continuous-flow, steady-state model evaporator Enthalpy-concentration diagram for sodium hydroxide-water system. Multiple-Effect Evaporator Systems When condensing steam is used to evaporate water from an aqueous solution, the heat of condensation of the higher temperature condensing steam is less than the heat of vaporization of the lower-temperature boiling water. consequently, less than 1 kilogram of vapor is produced per kilogram condensation of heating steam. This ratio is called the economy. To reduce the amount of steam required and, thereby, increase the economy, a series of evaporators, called effects, can be used. The increased economy is achieved by operating the effects at different pressures, and thus at different boiling temperatures, so that vapor produced in one effect can be condensed to supply the heat in another effect. Multiple-Effect Evaporator Systems 1. Forward-feed, triple-effect Multiple-Effect Evaporator Systems 1. Forward-feed, triple-effect This pattern of liquid flow is the simplest. One-third of the total evaporation occurs in each effect. To achieve a temperature-driving force for heat transfer in the second effect, the pressure of the second effect, P2, is lower than that of the first effect. This procedure is repeated in the third effect. For three effects, the flow rate of steam entering the first is only about one-third of the amount of steam that would be required if only one effect were used. the temperature-driving force in each of the three effects is only about one-third of that in a single effect. Therefore, the heat transfer area of each of the three evaporators in a triple-effect system is approximately the same as for the one evaporator in a single-effect unit. It requires a pump for feeding dilute solution to the first effect, since this effect is often at about atmospheric pressure, and a pump to remove thick liquor from the last effect. The transfer from effect to effect, however, can be done without pumps, since the flow is in the direction of decreasing pressure, and control valves in the transfer line are all that is required. Multiple-Effect Evaporator Systems 2. Backward-feed, triple-effect Backward-feed, triple-effect When the temperature of the fresh feed is significantly below its saturation temperature corresponding to the pressure in the first effect, backward-feed operation is desirable. The cold fresh feed is sent to the third effect, which operates at the lowest pressure and, therefore, the lowest temperature. Unlike the forward-feed system, pumps are required to move the concentrate from one effect to the next because PI > P2 > P3. Backward feed often gives a higher capacity than forward feed when the thick liquor is viscous, but it may give a lower economy than forward feed when the feed liquor is cold. Multiple-Effect Evaporator Systems 3. mixed feed Multiple-Effect Evaporator Systems 4. parallel feed CAPACITY AND ECONOMY OF MULTIPLE-EFFECT EVAPORATORS The total capacity of a multiple-effect evaporator is usually no greater than that of a single-effect evaporator having a heating surface equal to one of the effects and operating under the same terminal conditions, and, when there is an appreciable boiling-point elevation, is often considerably smaller. When the boiling-point elevation is negligible, the effective overall ΔT equals the sum of the ΔT's in each effect, and the amount of water evaporated per unit area of surface in an N-effect multiple-effect evaporator is approximately 1/Nth that in the single effect. Effect of boiling-point elevation on capacity of evaporators Effect of boiling-point elevation on capacity of evaporators Consider an evaporator that is concentrating a solution with a large boiling-point elevation. The vapor coming from this boiling solution is at the solution temperature and is therefore superheated by the amount of the boiling point elevation. Superheated steam is essentially equivalent to saturated steam at the same pressure when used as a heating medium. The temperature drop in any effect, therefore, is calculated from the temperature of saturated steam at the pressure of the steam chest, and not from the temperature of the boiling liquid in the previous effect. This means that the boiling-point elevation in any effect is lost from the total available temperature drop. This loss occurs in every effect of a multiple-effect evaporator, and the resulting loss of capacity is often important. The boiling-point elevation tends to make the capacity of a multiple-effect evaporator less than that of the corresponding single effect. In a single-effect unit producing 50 percent NaOH, for example, the overall coefficient U for this viscous liquid would be small. In a triple-effect unit, the coefficient in the final effect would be the same as that in the single effect, but in the other effects, where the NaOH concentration is much lower than 50 percent, the coefficients would be greater. Thus the average coefficient for the triple-effect evaporator would be greater than that for the single effect. The economy of a multiple effect evaporator depends on heat-balance considerations and not on the rate of heat transfer. The capacity is reduced by the boiling-point elevation. The capacity, on the other hand, is reduced by the boiling-point elevation. The capacity of a double-effect evaporator concentrating a solution with a boiling-point elevation is generally less than half the capacity of two single effects, each operating with the same overall temperature drop. The capacity of a triple effect is generally less than one-third that of three single effects with the same terminal temperatures. The optimum number of effects must be found from an economic balance between the savings in steam obtained by multiple-effect operation and the added investment required.