relative humidity. The dew point and wet bulb temperatures at... 14-90

14-49
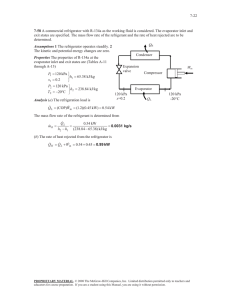
14-90 Atmospheric air enters the evaporator of an automobile air conditioner at a specified pressure, temperature, and relative humidity. The dew point and wet bulb temperatures at the inlet to the evaporator section, the required heat transfer rate from the atmospheric air to the evaporator fluid, and the rate of condensation of water vapor in the evaporator section are to be determined.
Assumptions 1 This is a steady-flow process and thus the mass flow rate of dry air remains constant during the entire process ( a 1
a 2
a
) . 2 Dry air and water vapor are ideal gases. 3 The kinetic and potential energy changes are negligible.
Analysis The inlet and exit states of the air are completely specified, and the total pressure is 1 atm. The properties of the air at the inlet and exit states may be determined from the psychrometric chart (Fig. A-31) or using EES psychrometric functions to be (we used EES)
T dp1
15.7
C
T wb1
19.5
C h
1
1
55 .
60 kJ/kg dry air
0 .
01115 kg H
2
O/kg dry air v
1
0 .
8655 m
3
/ kg dry air h
2
2
27 .
35 kJ/kg dry air
0 .
00686 kg H
2
O/kg dry air
T
2
2
=10
C
= 90%
2
Cooling coils
1 atm
Condensate
1
T
1
1
=27
C
= 50%
The mass flow rate of dry air is
10
C
Condensate removal a
V
1 v
1
V car
ACH v
1
( 2 m
3
/change)(5 changes/mi n)
0.8655
m
3
11 .
55 kg/min
The mass flow rates of vapor at the inlet and exit are v 1
1
a
( 0 .
01115 )( 11 .
55 kg/min)
0.1288
kg/min v 2
2
a
( 0 .
00686 )( 11 .
55 kg/min)
0.07926
kg/min
An energy balance on the control volume gives a h
1
out
a h
2
w h w 2 where the the enthalpy of condensate water is h w 2
h f @ 10
C
42 .
02 kJ/kg (Table A 4) and the rate of condensation of water vapor is w
v 1
v 2
0 .
1288
0 .
07926
0.0495
kg/min
Substituting, a h
1
( 11 .
55 kg/min)(55 .60
kJ/kg)
out
out
out
a h
2
w h w 2
( 11 .
55 kg/min)(27 .35
kJ/kg)
( 0 .
0495 kg/min)(42 .02
kJ/kg)
324 .
4 kJ/min
5.41
kW
PROPRIETARY MATERIAL . © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.
14-50
0.050
0.045
Pressure = 101.3 [kPa]
AirH2O
0.040
0.035
35°C
0.8
0.030
30°C
0.6
0.025
25°C
0.020
0.4
0.015
20°C
15°C
0.010
0.005
10°C
2
1
0.2
0.000
0 5 10 15 20 25 30 35 40
T [°C]
Discussion We we could not show the process line between the states 1 and 2 because we do not know the process path.
PROPRIETARY MATERIAL . © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.