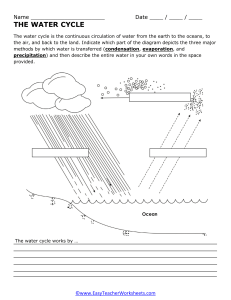
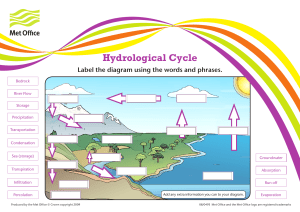
Date: July 3rd, 2023 Name: Revision CT2 Q1. List the properties of solids, liquids and gases. Solids Liquids Gases Q2. Use particle theory to explain how liquid changes to a gas. CLS 6 Q3. Draw particles in the following boxes to show particles in solid, liquid and gas. Q4. Define the following terms: 1. Freezing – 2. Condensation – Q5. Write two differences between boiling and evaporation. Boiling Evaporation Q6. Draw a well labelled diagram of water cycle showing the processes of evaporation, transpiration, condensation and precipitation. CLS 6 Q7. Write the symbols of following elements. 1. sodium 2. chlorine 3. potassium 4. argon 5. nitrogen Q8. Write the names of elements with following symbols. 1. Mg 2. P 3. Li CLS 6 4. B 5. Si Q9. What is the name of the compound when these elements are combined together? 1. magnesium and oxygen 2. potassium, oxygen and nitrogen 3. sodium and fluorine 4. calcium and chlorine Q10. Which elements are found in these compounds? 1. copper sulfate 2. sodium chloride 3. sulfur dioxide Q11. Calculate the weight of an object on Earth if mass of the object is 25 kg. The strength of gravity on Earth is 10 N/kg. Q12. Is mass same as weight? Explain. CLS 6