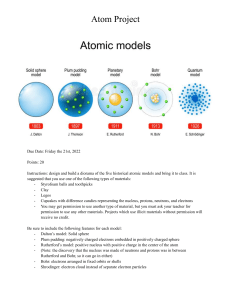
This was the first model of the atom ever proposed. It was simple and described atoms as tiny spheres that could not be broken down into smaller pieces. Democritus's model of the atom The "Plum Pudding Model" of the atom The "Rutherford Model" of the atom The "Quantum Mechanical Model" of the atom This model this model was the first to show a nucleus, consisting of protons and neutron. Electrons surround the nucleus but are not shown in distinct energy levels. The "Rutherford Model" of the atom The "Plum Pudding Model" of the atom The "Quantum Mechanical Modell" of the atom Democritus's model of the atom Who proposed that electrons move around the nucleus in circular orbits? Dalton Thomson Rutherford Bohr Who discovered that atoms are mostly empty space? Dalto Thomson Rutherford Bohr Which scientist proposed a new atomic model after discovering the electron in 1897? Dalton Thomson Rutherford Bohr Who proposed that electrons move around the nucleus in circular orbits? Dalton Thomson Rutherford Bohr This model added on to previous models by showing electrons existed at certain "energy levels". However it does not accurately show what those levels look like. The "Bohr Model" of the atom The "Rutherford Model" of the atom The "Plum Pudding Model" of the atom The "Quantum Mechanical Model" of the atom This model was developed after J.J. Thompson discovered electrons, a particle smaller than an atom. Is shows electrons floating freely in a positive space. The "Plum Pudding Model" of the atom The "Rutherford Model" of the atom Democritus's model of the atom The "Quantum Mechanical Model" of the atom What does the nucleus of an atom contain? Electrons & Neutrons Protons & Neutrons Neutrinos & Positrons Ernest Rutherford discovered that atoms were mostly _________________. negatively charged positively charged electrons empty space. In the gold foil experiment, most of the positively charged alpha particles passed through the gold foil, but some were deflected or bounced back. What did we conclude because of this? Atoms are small indivisible spheres Atoms are mostly empty space with a small, dense, positive center Atoms have negatively charged particles which orbit the nucleus Light is a wave, not a particle Idea was of an indivisible particle of matter he called "atomos" John Dalton Aristotle Democritus Plato He discovered the neutron in the nucleus and completed the current atomic model. J.J. Thomson Ernest Rutherford Niels Bohr James CHadwick He found that atoms have smaller parts and found negative electrons that he theorized were surrounded in a sphere of positive charges J.J. Thomson Ernest Rutherford Niels Bohr James Chadwick He found the positively charged protons in the nucleus and said that electrons moved around the nucleus J.J. Thomson Ernest Rutherford Niels Bohr James Chadwick He found that electrons have specific amounts of energy and move in specific orbits around the nucleus, like planets orbiting the sun. J.J. Thomson Ernest Rutherford Niels Bohr James Chadwick The atomic particle with no charge is the... proton neutron electron nucleus The atomic particle with a positive charge is the... proton neutron electron nucleus The atomic particle with a negative charge is the... proton neutron electron nucleus The smallest part of the element with the properties of the element is the... electron quark atom proton Which is an electron? A B C The Early model of the atom that states that all matter is made of atoms that are indivisible and indestructible. Dalton's Atomic theory Thomson’s Plumb Pudding Model Bohr’s Atomic Model Electron Cloud Atomic Model Describes possible locations of electrons around the nucleus. Dalton’s Atomic Theory Thomson’s Plumb Pudding Model Bohr’s Atomic Model Electron Cloud Atomic Model Suggested that negative electrons are stuck into a lump of positive charge. Dalton’s Atomic Theory Thomson’s Plumb Pudding Model Bohr’s Atomic Model Electron Cloud Atomic Model States that electrons move in spherical orbits around the nucleus. Dalton’s Atomic Theory Thomson’s Plumb Pudding Model Bohr’s Atomic Model Electron Cloud Atomic Model First model to have electron energy levels. Dalton’s Atomic Theory Thomson’s Plumb Pudding Model Bohr’s Atomic Model Electron Cloud Atomic Model What is the mass number of Hydrogen-2? 0 1 2 3 How many protons does 42He have? 2 3 4 6 How many neutrons does 126C have? 6 12 18 0 How many protons are in 158O ? 8 15 7 33 If an element has 7 protons and 7 neutrons, what element is it? Silicon Nitrogen Indium Sodium