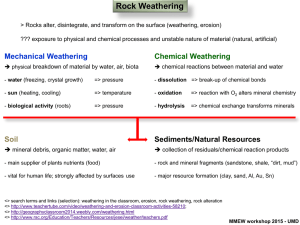
1 WEATHERING – BIPARTITE APPROACH AND BASIC PRINCIPLES 2022 GGY 168 Weathering Barend vd Merwe 1 1.1 Weathering – Bipartite Approach and Basic Principles Premises 1.1. Weathering is the chemical and physical alteration of rocks and minerals at OR near the Earth’s surface 1 . 1.2. The process of weathering leads to the development of more stable products 2 . 1.3. The transition from unstable reagent to stable product i very low 12 . 1.4. Weathering can be either physical, or chemical, or both physical and chemical 2 . 1.5. Atoms are connected to each other by bond. 1.6. The bonds between atoms can either be ionic OR covalent 12 . 1.7. An element is a substance consisting of a single type of atom 1 . 1.8. A compound is a substance that consists of two or more different elements 1 . 1.9. Rocks AND minerals consists of compounds 1 . 1.10. Physical processes lead to the breakdown of the original rock into smaller pieces 12 . 1.11. Chemical weathering leads to the production of secondary minerals 11 . 1.12. Water plays an important role in chemical weathering processes 12 . 1.13. Water plays an important role in physical weathering processes 12 . 1.14. Water is an effective solvent 12 . 1.15. Water can move downwards under the force of gravity 12 . 1.16. Water can move upwards due to capillary suction 12 . 1.17. Rocks have chemical properties 1 . 1.18. Rocks have bulk physical properties 1 . 1.19. Igneous rocks consist of interlocking crystals 1 . 1.20. It is not the case that sedimentary rocks contain interlocking crystals 1 . ©2022 University of Pretoria GGY 168 1 1.2 Rules 1 WEATHERING – BIPARTITE APPROACH AND BASIC PRINCIPLES Table 1: The influence of bulk physical properties on weathering (adapted from Bland and Rolls 1 ). Property Texture Discontinuities 1.2 Influence on weathering Influences rock strength and controls water availability and movement. It can increase the area that is in contact with water. It weaken the resistance of a rock to stress. Can contain weathered material which expands in the presence of water. Rules 1.1. IF two or more different elements are combined, THEN a compound is formed 1 . 1.2. IF the bond between two atoms involve the transfer of electrons, THEN an ionic bond is formed 1 . 1.3. IF the bond between two atoms involve the sharing of electrons, THEN a covalent bond is formed 1 . 1.4. IF the conditions under which a rock formed is different from those experienced at the earth’s surface, THEN the rock will experience weathering 2 . 1.5. IF a rock that formed in environment A is placed into environment B, where A and B are dissimilar, THEN the rock will adjust physically or chemically to the conditions of environment B 12;9 . 1.6. IF no chemical alteration of the rock occurs during this adjustment, THEN the rock is undergoing physical weathering 12;2 . 1.7. IF new and more stable chemical products are formed during the adjustment process, THEN chemical weathering is occurring 12;2 . 1.8. IF a particle is a clay particle, THEN it is a secondary particle 11 . 1.9. IF a mineral forms from the decomposition of another mineral OR If a mineral forms from precipitation, THEN the mineral is a secondary mineral 15 . 1.10. IF a mineral has not been altered since deposition OR IF a mineral has not been altered since crystallisation, THEN that mineral is a primary mineral 15 . 1.11. IF the size of particles within a rock decreases, THEN the total surface area of the particles increases 1 . 1.12. IF the total surface area of the particles in a rock increases, THEN the area of water-mineral interaction increases 1 . 1.13. IF a rock has a high porosity, THEN it can store a lot of water 1 . 1.14. IF a rock is contracting, THEN it experiences compressive stress 1 . (see Table 2 and Figure 1) 1.15. IF a rock is expanding, THEN it is experiencing tensile stress 1 . (see Table 2 and Figure 1) 1.16. IF forces are applied unevenly, THEN a rock experiences shearing stress 1 . (see Table 2 and Figure 1) Table 2: Stress (M N m−2 ) at failure for different types of rocks (adapted from Bland and Rolls 1 ). Rock Types Granite Dolerite Basalt Quartzite Sandstone Shale Limestone ©2022 University of Pretoria Tensile Stress 7–25 15–35 10–30 10–30 4–25 2–10 5–25 Compressive Stress 100–250 100–350 100–300 150–300 20–170 5–100 30-250 GGY 168 Shear Stress 14–50 25–60 20–60 20–60 8–40 3–30 10–50 2 1.2 Rules 1 WEATHERING – BIPARTITE APPROACH AND BASIC PRINCIPLES Compressive Tensile Shear Figure 1: The types of stress exerted on a rock (adapted from Bland and Rolls 1 ). Dashed lines indicate points of failure. ©2022 University of Pretoria GGY 168 3 REFERENCES REFERENCES References [1] Bland, W. and Rolls, D. 1998 Weathering: An introduction to the scientific principles. London: Arnold. [2] Dixon, J.C. 2004 Weathering. In: A.S. Goudie (Ed.) Encyclopedia of Geomorphology. London, UK: Routledge, pp. 1108–1112. [3] Ford, D. and Williams, P. 2007 Karst hydrogeology and geomorphology. West Sussex: John Wiley & Sons, Ltd. [4] Gillieson, D. 1998 Caves: Processes, development, and management. Oxford: Blackwell Publishers. [5] Hall, K., Lindgren, S. and Jackson, P. 2005 Rock albedo and monitoring of thermal conditions in respect of weathering: some expected and some unexpected results. Earth Surface Processes and Landforms, 30, 801 – 811. [6] Hefferan, K. and O’Brien, J. 2010 Earth Materials. West Sussex, UK: Wiley-Blackwell. [7] Kotz, J.C. and Treichel Jr., P. 1999 Chemistry and chemical reactivity. 4th edition. Fort Worth: Saunders College Publishing and Harcourt Brace College Publishers. [8] Leeder, M. and Pérez-Arlucea, M. 2006 Physical Processe in Earth and Environmental Sciences. Malden, USA: Blackwell Publishing. [9] Melosh, H.J. 2011 Planetary surface processes. Cambridge: Cambridge University Press. [10] ¡olaro, J.L. and McKay, C.P. 2010 Processes controlling rapid temperature variations on rock surfaces. Earth Surface Processes and Landforms, 35, 201–507. [11] Ryan, P. 2014 Environmental and low temperature geochemistry. West Sussex: John Wiley and Sons. [12] Summerfield, M.A. 1991 Global Geomorphology: An Introduction to the Study of Landforms. Harlow, UK: Pearson Prentice Hall. [13] Szokolay, S.V. 1996 Solar geometry. PLEA, Passive and Low Energy Architecture International in assoc. with Dept. of Architecture, University of Queensland Brisbane. [14] Tyson, P. and Preston-Whyte, R. 2000 The weather and climate of southern Africa. Oxford: Oxford University Press. [15] van der Watt, H. and van Rooyen, T. 1995 A glossary of soil science. Second edition edition. Pretoria: The Soil Science Society of South Africa. [16] Wu, J.C.S., Sheen, J.D., Chen, S.Y. and Fan, Y.C. 2001 Feasability of co2 fixation via artificial rock weathering. Industrial and Engineering Chemistry Research, 40, 3902–3905. ©2022 University of Pretoria GGY 168 4