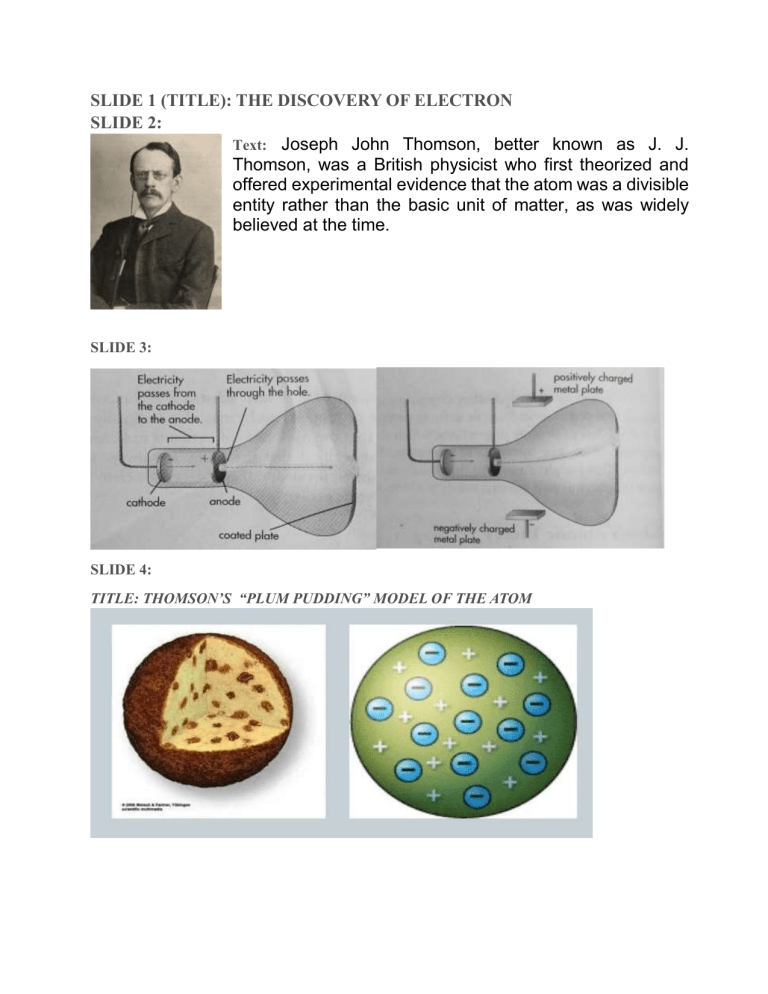
SLIDE 1 (TITLE): THE DISCOVERY OF ELECTRON SLIDE 2: Text: Joseph John Thomson, better known as J. J. Thomson, was a British physicist who first theorized and offered experimental evidence that the atom was a divisible entity rather than the basic unit of matter, as was widely believed at the time. SLIDE 3: SLIDE 4: TITLE: THOMSON’S “PLUM PUDDING” MODEL OF THE ATOM The Electron Electrons are negatively charged sub-atomic particles that help make up atoms. Electrons are found outside the nucleus in orbitals. The outermost orbital contains valence electrons (electrons that can be shared or exchanged with other atoms to form bonds). They were discovered in 1897 when J.J Thompson conducted his cathode-ray tube experiment. They have a mass of 0.00055 amu. So, they don't contribute to an element's atomic weight. About The Electron







