1. Apply knowledge of electron shells to find the valence of elements.
Show the valence electrons in an atom of:
a. Carbon
b. Nitrogen
c.
Neon
d. Sodium
2. Understand the difference between types of bonding and forces.
Explain whether covalent bonding is an intramolecular or intermolecular force.
Topic Notes
In this lesson, we will learn:
What is meant by the general term bonding and the categories of bonding.
Why different chemical substances display different types of bonding.
How different bonds and their varying strengths lead to different chemical properties.
The link between the bonding, structure and properties of chemical substances.
Notes:
There are less than 120 different types of atoms (the elements) in the periodic table, but there
are tens of millions of different chemical species. How is this possible?
In short, there is just an incredibly large number of different possible combinations and
arrangements of atoms. The ways an atom can combine to form larger, more organized
structures, and the attractive forces that make this happen is known as the bonding of the
substance.
There are several detailed theories of bonding, which try to explain things like:
o
o
o
Why atoms tend to combine into molecules in the first place. Why is it the case that
most elements are just more stable joined to other atoms than existing as individual
atoms? For a specific example, think of hydrogen existing as H2 molecules and not
lone H atoms.
Why bonding occurs in some species and not in others. Why does hydrogen exist as
H2 but helium gas is not He2?
Why some substances show completely different properties to others. Why does
carbon dioxide have a low melting point and poor conductivity, while carbon, as
graphite, has a high melting point and can conduct electricity?
You have seen simple answers to some of these already such as in Electronic structure: 288 rule,
where we learned that atoms like to have a full outer shell of electrons. We also learned about
electronegativity as the ability to attract electrons and complete a full outer shell.
These are simple explanations which work well for now. To start, remember that valence (the
number of outer shell electrons) and electronegativity heavily affect how atoms can bond.
This chapter will look at the different types of bonding we see in substances and how these lead
to varying structures and properties (like melting point, electrical conductivity etc) we observe.
We also look at how to predict when certain bonding will occur and how to represent it when
drawing molecules.
Bonding is a very general word. It can be used to describe any of the attractive forces that act
between or inside molecules, and if you are asked to describe the bonding in a substance you
should talk about any attractive forces present in the substance.
Before learning any types of bonding, recall the principles of electrostatic forces we saw in the
Periodic Table and Elements chapter. They will help explain why different types of bonding exist:
o #1: Oppositely charged particles attract each other, while particles of like charge
repel each other.
o #2: The greater the charge difference of two particles, the greater their force of
attraction (for example, the attractive force of a 2+ charge attracting a 2- charge is
greater than the attractive force of a 1+ charge attracting a 1- charge).
o #3: Attractive forces between oppositely charge particles decrease with distance.
o #4: Repulsive forces between like charged particles decrease with distance.
There are two broad categories of attractive forces (bonds) in chemical substances:
o
o
Forces that hold the atoms of a molecule or compound together, acting between the
atoms inside molecules, are intramolecular forces. All chemical bonds are
intramolecular forces.
Forces and interactions in between molecules are called intermolecular forces.
These are the forces that often determine if something is a gas, liquid or solid at
room temperature.
Intramolecular forces (ionic and covalent bonds) are much stronger than intermolecular forces.
When a substance melts or evaporates, it is the intermolecular forces being overcome, not the
intramolecular forces!
It is important to understand the difference now:
o
o
Different substances (carbon monoxide and carbon dioxide) are made by
rearranging atoms WITHIN a molecule. This would be different intramolecular
forces.
Different phases of a substance (carbon dioxide gas or liquid carbon dioxide) are
made by overcoming the intermolecular forces BETWEEN molecules. This is to say:
In a solid, the attractive forces holding the molecules together have not
been overcome. This is why the particles are densely packed and have
little space between them.
In a liquid, the intermolecular attractive forces have been partially
overcome. This is why substances in liquid phase can flow and are
usually less dense than their solid phase.
In a gas, the intermolecular forces have been completely overcome.
This is why gases flow extremely easily and the particles, supplied with
a lot of energy, are very energetic and gases take up a lot of volume
compared to their liquid or solid phases.
All this time, changing the phase is not changing the identity of the substance at all. If the bonds
within the molecules are unchanged, the chemical substance does not change identity.
How an atom bonds is mostly determined by the valence of the atom; this is the number of
unpaired outer shell electrons. The valence practically tells you how many bonds the atom can
make:
o In Electronic structure: 288 rule and Electronic structure: Subshells, we saw that the
shape of the Periodic Table is made to show the different electron subshells or
orbitals that atoms have the s, p, d block etc.
o To find the number of valence electrons in the outer shell, show the outer shell
as four equal orbitals, not separate s and p sections. We will find out why later. Fill in
the valence shell by adding single unpaired electrons to the four orbitals, then start
pairing them up with the 5th onward.
o Because paired electrons generally dont bond, only the unpaired electrons are
available to make bonds these are the valence electrons.
o
Across groups 1-8 (ignoring the d-block), the valence of the groups are: 1, 2, 3s, 4, 3,
2, 1, 0. The higher the valence of an atom, the more bonds it can make. This comes
from the outer shell electron configuration. Being comfortable with electron
configurations will help your understanding of bonding a lot!
The properties a chemical displays are due to the types of bonding and interactive forces
between its atoms and molecules, and the types of bonding a chemical shows is because of its
atoms' valence, and the electrons being able to make certain bonds in order to gain a full valence
shell.
o
Example 1: Neon is a noble gas with 8 valence electrons all paired up. Therefore
neon atoms have a valence of zero, and they don't make bonds between atoms to
form molecules or compounds.
This means they don't have strong intramolecular forces; a sample of
neon gas exists as millions of single atoms freely floating in space.
o
Therefore they are entirely limited to weak intermolecular forces and
so have very low boiling points, are gases at room temperature and
cannot conduct heat or electricity.
Example 2: Carbon atoms have four unpaired valence electrons, meaning carbon
atoms have a valence of four and each atom can make four covalent bonds (a type
of intramolecular force) with other atoms.
Because each atom can 'connect' with a strong bond to four others,
pure carbon is found in some forms that have the atoms in a single
giant structured network, made of millions of individual carbon atoms
all covalently bonded together. This is the structure of diamond.
When doing this, because the structure is effectively one giant
molecule and no one atom can be disturbed without disrupting the
entire structure, diamond is the hardest substance known to man and,
practically speaking, cannot be melted.
The valence bonding and interactive forces that exist in a chemical species gives rise to
the structure of the molecules it makes, which dictate its properties. Understanding the link
between bonding that leads to structure that leads to properties is crucial and will allow you to
make some predictions about certain chemical substances even when given just the formula
1. Identify whether covalent or ionic bonds will form between these elements.
Will the following pairs of atoms form a covalent or ionic compound? Explain why.
i) P and O
ii) Na and O
iii) C and Cl
iv) N and Cl
2. Identify covalent or ionic compounds and predict their formula using valence.
Predict whether these atoms will form a covalent or ionic compound together, then predict
the formula of their compound:
i) Mg and O
ii) C and O
iii) P and Cl
iv) Na and F
Topic Notes
In this lesson, we will learn:
The two major types of bonding in chemical compounds
To explain why both types exist and when they are likely to form.
The varying properties of ionic and covalent bonding.
How to predict the formula of ionic and covalent compounds.
Notes:
In Introduction to bonding, we saw the two broad categories of bonding: intermolecular forces
(the forces acting between molecules) and intramolecular forces (the bonds within molecules).
Molecules of a substance are held together by intramolecular forces - chemical bonds between
the atoms that the substance is made of.
o
For example, CO2 is carbon dioxide and it is carbon dioxide because it is made of one
carbon atom bonding to two oxygen atoms, sitting between them in the middle of
the molecule. If this arrangement changes, CO2 changes into something else.
We will not use intramolecular forces anymore. They are chemical bonds, or just bonds.
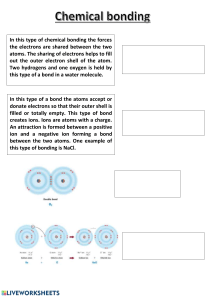
One major type of chemical bond is the ionic bond:
o Ionic bonding is a bonding attraction caused by oppositely charged ions interacting
with each other. It occurs between metal and non-metal atoms.
o To obtain a full outer shell, metal atoms can donate their outer shell electrons to
non-metal atoms. Having lost electrons, the metal atom has become a positive ion
and the non-metal atom that gained them is now a negative ion.
o The transfer of electrons is driven by the stability of having a full outer shell and the
difference in electronegativity. The non-metal atom will have a much higher
electronegativity value than the metal atom.
o The force of attraction in an ionic bond is the oppositely charged ions
interacting with each other. Electrostatic principles apply: a 2+ metal ion with a 2nonmetal ion is a stronger ionic bond than 1+ with a 1-.
o Examples of simple ionic compounds are sodium chloride, NaCl, or magnesium
oxide, MgO.
o Ionic bonds form between elements on opposite sides of the periodic table.
Periodic trends like atomic radius and the charge of ions will influence ionic bond strength in
compounds. These can be explained in terms of electrostatic theory.
o
Ions with a smaller atomic radius enable any oppositely charged ions to come into
closer contact, which increases ionic bond strength. In other words, opposite
charges make a stronger bond when they are closer to each other.
o
The larger the charge on an ion, the greater the charge difference will be with the
oppositely charged ion, which produces a stronger ionic bond. 2+ will attract 2stronger than 1+ attracts 1-.
Nuclear charge affects atomic/ionic radius and it helps explain trends seen in isoelectronic ions
(ions with the same number of electrons, e.g. N3-, O2-, F-, Na+, Mg2+, Al3+). In a group of these ions,
the ion with the greatest nuclear charge has the smallest atomic radius because the increasingly
positive nuclear charge attracts the same number of electrons more strongly.
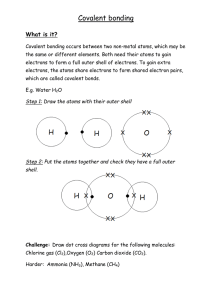
Another major type of chemical bond is the covalent bond:
o Covalent bonding happens when two atoms share a pair of electrons between them.
In any electron pair that make a covalent bond, one electron comes from each atom,
hence the name co (cooperating, working together) valent.
o Covalent bonding occurs between non-metal atoms with similar electronegativity.
The similar electronegativity is what leads to the electrons being shared, not gained
or lost as in an ionic bond. Because covalent bonding involves
atoms gaining electrons by sharing them to complete an outer shell, it is very rare to
see metal atoms covalently bond.
o The driving force of a covalent bond forming is that both atoms have more
completion of their outer shell.
o The force of attraction in a covalent bond is the shared electron pair being attracted
to the nuclei of both atoms making the covalent bond.
o
o
Covalent bonding can involve more than one electron pair:
A covalent bond with one electron pair is a single bond.
A covalent bond with two electron pairs is a double bond and is almost
twice as strong as a single bond.
A covalent bond with three electron pairs is a triple bond, almost three
times as strong as a single bond.
Well see why its almost twice/three times as strong later.
Covalent bonds can vary in length (measured by the distance between the two
nuclei), but in general the stronger the bond, the shorter the bond length.
Covalent bonding requires similar, not equal electronegativity. There can be some variety in this
electronegativity gap which creates dipoles, or polar covalent bonds. It is not a black and white
issue whether you have a covalent or ionic bond; it is a dial which can be turned from 0 to 100.
o
Start by knowing that covalent bonds occur between metal and nonmetal atoms.
o
The larger the gap in electronegativity between two atoms, the more polar their
covalent bond will be. A polar bond means the two atoms making the bond have
opposing partial charges (\deltaδ+ or \deltaδ-). They are not full ions; the atoms
are just not sharing the electrons equally because the more electronegative atom
has a greater pull on them.
o
Because of the opposite partial charges, we have created something of a
north/south pole along the bond. We call this a dipole. It is not an ionic bond, but
a polar covalent bond. We can also say this covalent bond has slight ionic character.
o
This slightly ionic character means that the two atoms are slightly attracted to one
anothers opposing charges, like an ionic bond - at least more than if there was zero
ionic character!
For this reason, bond polarity tends to make covalent bonds stronger.
Lets take two examples to show the difference:
o
When carbon bonds with bromine, there is a small gap in electronegativity where
bromine pulls the electrons with greater force than carbon. This means there is only
a very slight partial charge on the two atoms and the electrons are almost equally
shared. The relatively bulky bromine atom also makes the covalent bond long; the
carbon and bromine nuclei are relatively far away from the electron pair they share.
This adds up to a weak C-Br covalent bond with very little polarity.
o
When carbon bonds with fluorine, there is a very large electronegativity gap.
Fluorine pulls the electrons toward itself substantially more than carbon does. This
creates large partial \deltaδ+ and \deltaδ- charges on carbon and fluorine
respectively. The small size of the fluorine atom also means the atoms can be in
closer contact and the attractive forces are stronger.
This all adds up to a very strong C-F covalent bond with significant ionic character.
When covalent compounds contain polar bonds, the unequal pull of electrons towards some
atoms and away from others creates polar molecules. We say that these molecules
have permanent dipoles. These are represented with vector arrows that point from the positive
to areas of negative charge.
Polarity in molecules has a major effect on intermolecular forces and the solubility of a
substance, which we will see in Intermolecular forces and Polarity.
A gap in electronegativity is the easiest way to predict whether ionic or covalent bonding will
occur in a chemical substance since it is electronegativity that drives the tendency for electrons
to be gained or lost.
o
When two atoms with a large gap in electronegativity form a bond, the atom with a
higher electronegativity is going to pull a lot more electron density (think of the
electrons as clouds).
Covalent and ionic bonds occur within molecules, between the atoms and ions that bond to
make a substance what it is. They are both strong forces simple ionic compounds often have
melting points of several hundred degrees Celsius, as do some large covalent structures.
You can use the valence of an atom to work out the formula of covalent and ionic compounds:
o For each atom, find the valence (number of unpaired) electrons. This will be the
group number up to carbons group. After this, electrons pair up in the outer shell,
so it will be 8 minus the group number. This is the valence of the atom. (E.g. N has a
valence of 8 - 5 = 3 due to 3 unpaired electrons, H has 1).
o Cross the valence of each atom with the other this is the number of atoms of the
element that will combine to form the compound. (N = 1, H = 3 makes NH3, a
covalent compound).
o
Example 1:
Remember to take the lowest whole-number ratio of atoms e.g. in the C2O4 example,
this can be simplified to CO2, or carbon dioxide.
Example 2:
(IB) Some covalent bonds are made by both electrons being donated by the same atom. This is
a coordinate covalent bond. They are normally made by lone pairs on atoms like oxygen or
nitrogen donating to H+ or a metal atom.
o For example, in the H2O molecule there are two lone pairs of electrons on oxygen
that are not making regular covalent bonds to the hydrogen atoms. These lone pairs
can form a covalent bond with a hydrogen ion in solution:
H2O + H+ \,→\, H3O+
This reaction happens whenever an acid is dissolved in water.
1. Identify the intermolecular forces present in molecules.
Look at the formula of the chemical substances listed below. Which type of intermolecular forces
would you expect to see between the molecules?
a. i) CH_44
ii) H_22O
b. i) Br_22
ii) HBr
2. Recall the order of intermolecular force strength
Look at each group of chemical substances below. Order them in how relatively strong their
intermolecular forces are. Hint: Identify the intermolecular forces present first.
a. F_22, I_22, Br_22, Cl_22
b. HCl, HF, HBr, HI
c.
NH_33, H_22O, CH_44
Topic Notes
In this lesson, we will learn:
To apply the principles of electrostatic forces to understand intermolecular forces.
To explain how hydrogen bonding, London forces and dipole-dipole interactions occur.
To recall the relative strength of relative forces between molecules.
Notes:
The molecules of chemical compounds are held together by chemical bonds, or forces between
the atoms the molecules are made of. As we saw earlier, these forces between atoms are called
intramolecular forces and the two major types, like two ends of a left-to-right spectrum, are
covalent and ionic bonding.
As well as intramolecular forces, there are intermolecular forces that occur between the
molecules of any chemical substances. Intermolecular forces are not as strong as intramolecular
forces, but they influence a lot of properties in a chemical. For example, the melting point of a
substance is greatly influenced by the intermolecular forces holding molecules together. To
summarize, we have:
o Intramolecular forces, which are strong and hold the atoms within a molecule
together. These are generally only broken in chemical reactions.
o The focus of this lesson: intermolecular forces, which are weaker but hold separate
molecules together. These can generally be overcome by physical changes such as
temperature.
We now need to understand what a dipole is, because all intermolecular forces are based on
dipole interactions. A dipole (di-pole, ‘two poles’) is a charge difference between atoms in part of
a molecule, created by excess charge. Whenever an atom or molecule is more positive than
another atom or molecule (which is more negative), you might have a dipole. This can happen in
a few ways.
o
Dipoles can form from a covalent bond between two elements of different
electronegativity.
Example below: carbon and chlorine can make a covalent bond.
Because chlorine is more electronegative than carbon, the electron pair
they share in the bond is going to be held closer to chlorine (on
average) than carbon. This means that this part of the molecule on
average is going to be slightly more negative (because the negativelycharged electrons are closer) around the chlorine atom than the carbon
atom, where the relative lack of electrons leaves carbon with a slight
positive charge. Therefore we give carbon a \deltaδ+ (delta positive)
and chlorine a \deltaδ- (delta negative) charge. These are ‘partial’
positive / negative charges.
Because the electronegativity of an atom does not change, this effect
will always be present in a carbon-chlorine bond – dipoles like this are
called permanent dipoles. Any bond between two atoms with a
difference in electronegativity creates a dipole like this. We call the
bond a polar bond because it has the positive/negative charged effect
of a north/south pole.
A major type of intermolecular force are van der Waals forces:
o One type of van der Waals forces are dipole-dipole interactions.
When a molecule of a compound has a permanent dipole (see above),
the delta positive ( \deltaδ+) and delta negative ( \deltaδ-) charge
influences other molecules around them too. This includes other
identical molecules!
Because each molecule has the same permanent dipole, they arrange
with the opposite ends of other molecules to maximize
their \deltaδ+/ \deltaδ- attractive forces with as many molecules as
possible across 3d space. See below for an example with HCl, where H
holds a \deltaδ+ and Cl holds a \deltaδ- charge in their covalent bond
together.
o
Because this effect is caused by dipoles across molecules interacting, it
is called dipole-dipole interactions. This is the stronger of the two van
der Waals type forces.
Another type of van der Waals forces are London dispersion forces (London forces).
Because electrons repel each other and are attracted to positive
charge, if two atoms or molecules approach one another, the electrons
in each will repel the other. (See below: the solid black is high electron
density, spotted is less electrons / lower density).
The movement of electrons due to repulsion polarizes both atoms and
leads to a dipole being created: see above.
This 'forced' polarization of two atoms/molecules that come too close
to each other is called an induced dipole. This is the attraction in
London dispersion forces.
Because this attractive force only exists when atoms/molecules are
close together, and vanishes if they are moved apart, it is a temporary
dipole, not a permanent one. For this reason we say London forces
are temporary induced dipole forces. They are weaker than permanent
dipole-dipole interactions (see above) and can exist between any
atoms.
London forces are stronger on atoms that are more polarizable –
meaning the charge (electrons) on the atom can be manipulated and
moved around. This occurs much more easily in larger atoms with more
electron shells (so the outer shell is further from the nucleus).
So, smaller atoms with less electron shells are less polarizable and have
weaker London forces. Think of the difference between trying to twist
and change the shape of a hard, small marble and a large bean bag –
the bean bag is a more ‘polarizable’ larger atom!
This explains why the melting point of helium is lowest in the noble
gases, and melting point increases going down the group as London
forces become stronger, needing more energy to overcome.
Another type of intermolecular force is known as hydrogen bonding. These are the strongest
intermolecular forces.
o
Hydrogen bonding is a very strong form of dipole-dipole interactions that
happens when hydrogen, H, covalently bonds to a very electronegative element ‘X’
(X = F, O or N). Because hydrogen is not very electronegative, these bonds make
strong permanent dipoles; the \deltaδ+ charge on hydrogen is relatively big, and
the \deltaδ- on the electronegative atom ‘X’ is equally strong.
o
This causes attractive forces; interactions between the negative lone pair electrons
on X in one molecule and the slightly positive hydrogen atom in the other. Chemists
say the lone pair donates a hydrogen bond and the hydrogen atom accepts
it. Hydrogen bonds are very similar to dipole-dipole interactions but even stronger.
o
The properties affected by hydrogen bonding are cumulative; the more hydrogen
bonding there is, the more the properties will are affected. Electronegative atoms
with more than one lone pair (like oxygen) can donate more than one hydrogen
bond, which affects properties more than if it could donate just one hydrogen bond
(e.g. nitrogen).
Intermolecular forces are cumulative – they have an 'adding up' effect and will occur across all or
any fraction of a molecule that makes contact with another. This is why:
o
Longer hydrocarbon chains (with ‘more molecule’ to make contact) have a higher
melting/boiling point than shorter ones.
o
Unbranched straight hydrocarbon chains (which can get closer to one another, so
they share more van der Waals forces) have a higher melting/boiling point than
branched chains which can’t make as much contact.
Water is a good example of how the strength and amount of hydrogen bonding in a chemical
structure can change properties considerably:
o Compared to similar compounds with the same number of electrons, water has an
unusually high melting/boiling point.
o
With two electronegative lone pairs and two electropositive hydrogen
atoms, it can donate two and accept two hydrogen bonds. With four
hydrogen bonds per molecule in total, intermolecular forces are much
stronger than in ammonia (only one lone pair and N-H being a weaker
dipole) and methane (with no lone pairs and therefore no hydrogen
bonding).
Liquid water has a higher density than solid ice. That’s why icebergs float at the
water’s surface!
As a solid where hydrogen bonding dictates how the water molecules
are ‘packed together’, the molecules are actually less compact than
when they are liquid. This makes ice less dense and so it floats on the
water’s surface
A chemical substance’s solubility depends on the intermolecular forces it displays. This is
important in planning chemical reactions. Generally, “like dissolves like” in terms of
intermolecular forces between reactants and solvents. Some important points about solubility
and intermolecular forces are below:
o The dipoles in water molecules are strong enough to dissolve some ionic
compounds by interacting with the individual ions. The \deltaδ- oxygen atoms can
interact with positive ions, and \deltaδ+ hydrogen atoms with negative ions. As
solvent (water) molecules far outnumber ions, Multiple water molecules can
surround ions in a process called hydration. This is why many simple ionic
compounds are soluble in water.
o
o
Alcohols such as ethanol are capable of hydrogen bonding, because of the O-H
group present. Ethanol molecules will interact strongly with water molecules
through their hydrogen bonding, so small simple alcohols such as ethanol are highly
soluble in water through mutual hydrogen bonding.
Not all polar molecules are soluble in water. Most haloalkanes for example have
permanent dipoles due to the carbon-halogen bond. However, any dipole-dipole
interactions they may have with water will not be strong enough to overcome
interactions between hydrogen-bonding water molecules interacting among
themselves. As such, haloalkanes are only slightly soluble in water because they
cannot form the hydrogen bonds necessary to interact with a water solvent.
The stronger or more intermolecular forces there are, the more the physical properties are affected –
hardness, melting and boiling point and whether a substance conducts electricity are all affected by
intermolecular forces. Later, in Polarity, you'll be able to predict these properties of substances based on
intermolecular forces!
1. Draw Lewis structures for simple covalent compounds.
Draw dot and cross structures for the following molecules:
a. HCl
b. N_22
c.
NH_33
d. AlCl_33
e. NH_{4}4+
2. Draw Lewis structures for simple ionic compounds.
Draw dot and cross structures for the following molecules:
a. KCl
b. Na_22O
c.
CaCl_22
Topic Notes
In this lesson, we will learn:
To construct Lewis (dot and cross) structures using knowledge of electrons shells and the octet
rule.
To use Lewis diagrams to explain the difference between ionic and covalent bonding.
Some of the exceptions to the octet rule and the significance of this to theories of bonding.
Notes:
In C11.4.2: Ionic and covalent bonding we saw the two main types of chemical bond: ionic and
covalent bonding. These are attractive forces that hold individual atoms together allowing them
to form molecules and larger, more complex structures.
We can obviously see that most atoms are just more stable existing in compounds or molecules,
such as CH4 or O2, compared to being single isolated atoms, like H or O.
In C11.4.1: Introduction to bonding we mentioned that theories of bonding exist to try to
explain why this is true. One theory of bonding is valence bond theory, which uses Lewis
structures to show the bonding in simple molecules.
In Lewis structures, dots and crosses are used to show the valence electrons, where dots or
crosses are used for the central atom and the other is used for the atom(s) it is bonding to. They
can be used to show covalent and ionic bonding between atoms in molecules and compounds,
where electrons are shared or donated/accepted to achieve a full outer shell. Valence means
outer shell or highest energy electrons; dont draw core electrons in Lewis structures!
o
When drawing these diagrams, look at our lesson Electronic structure: 288 rule for
help when finding the number of outer shell electrons. Remember the octet rule,
where electrons will try and fill their outer shell with an octet of (eight) electrons.
Lewis structures for covalent compounds can be drawn step-by-step as below, with CO_22 and
H_22O): as examples:
o
Step 1: Write the chemical symbol for the central atom in the molecule and draw a
ring around it (this will be the outer electron shell). If the molecule is diatomic, draw
either one atom.
CO2:
H2O:
o
Step 2: Write the symbols of the other atoms equally spaced around it. Draw a ring
around each of these so that their rings each have an overlap into the central ring.
These rings are the outer electron shells.
CO2:
H2O:
o
Step 3: For the non-central atoms, fill in their outer shells of electrons using the 288
rule fromElectronic structure: 288 rule (288 rule). Remember to fill up to four
electrons individually then start pairing them up. Mark all these electrons using
either a dot or a cross. Be consistent with the dots and crosses; if you use crosses for
one H atom, use it for all H atoms.
CO2:
H2O:
o
Step 4: LOOK CAREFULLY AT YOUR DIAGRAM. Now make sure any unpaired
electrons are placed in the overlap with the central atom.
This is a clue to show how many pairs of electrons (how many covalent bonds) there
will be with the central atom. Each of these will be paired with an electron from the
central atom too.
CO2:
H2O:
o
Step 5: Now fill the outer shell of electrons in the central atom. Use the other sign to
the one you used with the non-central atom. Start by making pairs of dots and
crosses with the electrons of the non-central atoms in the overlapping area. You can
only pair dots and crosses in a 1:1 ratio. Now, any extra electrons can be placed
outside the overlaps on the outer shell. Remember that if there are more than four
electrons in the shell they must now be paired. Electrons in the overlapping area
count for both overlapping atoms!
o
Step 6: Look carefully at your diagram,. If you have followed these steps, all of your
atoms should have exactly 8 electrons (2 if it is hydrogen) in the outer shell.
Remember that electrons in the overlaps count for both atoms.
CO2:
H2O:
Dot and cross diagrams for ionic compounds can be drawn like this:
o
Step 1: Write the chemical symbols of the atoms side by side and draw a ring (the
outer shell) around them but keep them separate. They must not overlap like in
covalent compounds.
o
Step 2: Fill the outer shell with the correct number of electrons for each atom.
NaCl example:
o
Step 3: Move the electrons from the metal atom(s) to the non-metal atom(s). This
should complete the outer shell of the non-metal atom(s) while leaving empty the
outer shell of the metal atom(s). The atoms are now ions.
o
Step 4: Ions should have square brackets surrounding them with their charge on the
top-right corner. This shows an ion has been formed. In order to show an electron
was transferred and not shared, keep the original dot/cross signage you used. See
below, where the Na outer electron is still a dot on Cl.
NaCl example:
These Lewis diagrams should help to show the difference between bonding in covalent and ionic
bonding in terms of electrons:
o
Covalent bonding involves a sharing of electrons to complete outer shells in both
atoms.
o
Ionic bonding involves a discrete transfer of electrons usually from a metal atom to a
non-metal atom, which also leads to complete outer shells.
Building Lewis structures using the octet rule is applying a theory of chemical bonding. These
theories are made to try and explain what we observe and to make predictions that can be
tested, but there are exceptions to the octet rule.
For example, the molecules NO (nitric oxide) and BF3 (boron trifluoride):
o
Nitric oxide (NO) would have a Lewis structure beginning with six electrons around
oxygen, and five electrons around nitrogen. This means nitrogen needs three more
electrons and oxygen two to satisfy the octet rule. This cannot be satisfied by:
o
A single bond (one pair of electrons) between the two atoms; it leaves
only six electrons on nitrogen and seven on oxygen.
A double bond (or two pairs of electrons) between the two atoms; it
leaves only seven electrons on nitrogen and eight on oxygen.
A triple bond between the two atoms; oxygen now has nine electrons
in the outer shell, violating the octet rule.
Boron trifluoride (BF3) would have a Lewis structure beginning with three electrons
around boron and seven around all three fluorine atoms each. The octet rule cannot
be satisfied for boron. It has only three electrons, and with one covalent bond each
to the three fluorine atoms, it is still has only six electrons when it should have eight.
If the theory behind Lewis structures was complete and totally accurate, neither of these two
molecules should exist. The fact that they do shows the theory is not totally accurate!
1. Predict the molecular geometry and bond angles of the following molecules:
a. i) Cl2
ii) AlCl2
b. i) CH4
ii) NH3
2. Predict the molecular geometry and bond angle of the following molecules
a. i) PCl5
ii) SF6
b. H2O
c.
CIF3
Topic Notes
In this lesson, we will learn:
To understand the ball-and-stick method of describing molecules in 3d space.
To understand the principles of VSEPR theory.
To apply VSEPR theory to predict the shapes and bond angles of different molecules.
Notes:
Molecules and compounds alike are groups of atoms held together by chemical bonds between
them. One way to imagine these molecules is a ball and stick model the atoms are spherical
balls connected by sticks (the chemical bonds) to each other to form the molecule.
Think about the following:
o Molecules are made of atoms connected by covalent bonds.
o A covalent bond is a pair of electrons shared between two atoms. The electrons
have a Coulombic attraction to the two nuclei they sit between.
o
Electrons repel each other. Electron pairs of any sort will try to place themselves as
far away from each other as possible to reduce repulsion.
We can use these to predict the shape of molecules by finding the number of valence (outer
shell) electrons the central atom has around it. This can determine both the shape and bond
angles around the central atom. This method is known as Valence Shell Electron Pair Repulsion
(VSEPR) theory.
According to VSEPR, the electron domain is what determines the geometry (shape) of a
molecule.
There are two contributing factors to the electron domain:
o The number of bonding electron pairs around the central atom. These outer shell
electrons will position themselves as far away from each other as possible to reduce
electron repulsion. Double and triple covalent bonds count as one electron domain.
For example, if there were two pairs of bonding electrons around one
atom, they would position themselves on opposite sides of the central
atom. The angle between the bonds they make would be 180°, which
we call the bond angle. The atoms of the molecule would be in a
straight line with each other, so the shape is called linear. See below for
CO2, a linear shaped compound with two bonding pairs of electrons.
If there were three pairs of bonding electrons around the central
atom they would divide the same 360° area in three equally spaced
angles – 120° each. The three directions the bonds now point and place
the atoms they're bonded to form the edges of a flat triangle, so the
shape is called trigonal planar. See below for BF3, a trigonal
planar molecule with three bonding pairs.
o
The number of pairs of non-bonding (lone pair) electrons around the central atom.
Non-bonding pairs cause greater repulsion than bonding pairs because the electrons
are localized on one atom rather than being shared by two atoms.
This means that bond angles in molecules with lone pairs are decreased – around
2.5° for each lone pair present.
Water, H2O, has two bonding pairs and two non-bonding pairs on the
central O atom. The normal bond angle of 109.5° for 4 bonding pairs
(see below) is reduced by 5° for the angle between the two bonding
pairs. The drop from 4 to 2 bonding pairs also means the shape changes
theres two less atoms to make a fewer with! Both lone pairs repel the
bonding pairs down into an angular or bent V shape. See below for the
geometry of water, H2O.
Molecular shapes and their bond angles can be predicted from finding the numbbyf bonding and
lone pairs around a central atom. Many shapes are summarized in this table:
In this lesson, we will learn:
To describe the features of metallic bonding and the structure of pure metals.
How to explain the properties of metals using metallic bonding theory.
How to explain the trends in properties of metals using metallic bonding theory.
Notes:
We saw earlier that ionic and covalent bonding are bonding types that hold compounds and
small molecules together, but in elemental metal samples (pure metals, not metal compounds)
there is a third type of bonding called metallic bonding.
In elemental metals (that means pure iron metal, not iron compounds), metallic bonding creates
a structure with the following features:
o
o
There is a lattice of positively charged metal ions.
Between these positive ions, there is a sea of negative delocalised electrons. These
are the electrons that the metal atoms (that are now ions!) had lost, so they could
gain a full outer shell.
The attractive force that keeps the structure together is the positive/negative electrostatic
attraction between these two features.
See the diagram below but remember that the electrons are freely moving, thats why they look a
bit disorganised.
Metallic bonding, occurs in samples of metal-only atoms, including pure metallic samples, and
explains the properties of pure metals that we observe.
o Metallic bonding occurs in PURE METAL SAMPLES. It occurs in alloys too, which are
mixtures of different metals we will look at next lesson.
o Metallic bonding does NOT occur in metal compounds with non-metal atoms. That
is ionic bonding which we learned in C11.4.2: Ionic and covalent bonding
As with any bonding theory, we use our ideas of metallic bonding to help explain the properties
that we see when we study metals. Metallic bonding explains the properties of metals in the
following ways:
o
o
o
There is a strong electrostatic attractive force between the metal ions and the
delocalised electrons. It takes a lot of energy to overcome this force and pull the
positive ions apart from the delocalised electrons. This is why most metals have a
high melting point.
The sea of delocalised electrons is fluid. This means the metal ions can
move amongst and around each other because they arent rigidly stuck in one place
in the lattice. This is why many pure metals are both malleable and ductile:
A material that is malleable can be bent and re-shaped when it is
heated up. This is how blacksmithing works (how swords and iron tools
are made), as hot metal is hammered into different shapes before it
hardens as it cools.
A material that is ductile can be bent and drawn into thin wires. Copper
is very ductile and most electrical wires are made from it.
The fluid sea of negatively charged delocalised electrons easily carry electric charge
and heat energy throughout the lattice.
This explains why metals are good conductors of both electricity and
heat.
There are trends in properties of metals, like their melting point. The trends are caused by
different metallic bonding strength which is caused by two main factors:
o
The charge of the metal ion in the lattice. For example, compare group 1 metals that
have a 1+ ion charge and group 2 metals with ions of 2+ charge. If you compare a
group 1 and group 2 metal in a period like Na and Mg, the group 2 metal will have a
higher melting point because of the greater charge difference.
In short, 2+ attracting 2- is a stronger force than 1+ attracting 1-.
o
The ionic radius of the ion. In Periodic trends: Atomic radius, we saw that the ionic
radius gets larger going down a group in the periodic table.
This means the nucleus (where the positive charge is) is further away from the
delocalised electrons it is attracted to, so a larger ionic radius makes a weaker
metallic bond. You can see this in the melting and boiling points of metal elements
decreasing down the group column.
Metallic structure and bonding is not only seen in pure metals, but also in mixtures of different
metals combined ‐ these are called alloys. Alloys are made to obtain unique or more precise
properties of two or more metal elements. We will look at alloys in our next lesson, Alloys.
Topic Notes
In this lesson, we will learn:
The definition of an alloy and how alloys and metals are related.
To explain the benefits of using metal alloys over pure metal substances.
To explain how the structure of an alloy gives it unique properties to its constituent metals.
Notes:
As seen in Metallic bonding, the properties we see in pure metals are explained by the features
of metallic bonding and the structure that it creates.
o
Remember what scientists are doing here. We study metals and find their properties
such as high melting point, they are malleable etc. THEN we create the theory of
metallic bonding that explains these properties and predicts others that we can test.
A lot of every day uses of metals rely on these properties:
o
o
Strength, which is the ability to withstand a force without breaking;
Hardness, which is the resistance to being deformed (having its shape broken).
However, many of these metals we use in real life situations are not pure metals because pure
metals have relatively low strength and hardness.
Remember metallic bonding: in pure metals, the ions arent stuck in place, so if you apply
pressure (like putting a lot of weight on it) pure metals dont hold up very well. Under very heavy
weight loads they will change shape and fail structurally over time. In practice, this means that
pure metals are not very useful in the most physically demanding of structures, such as in
buildings or bridges.
Alloys are mixtures of two or more metals (or carbon) combined to enhance properties.
o Alloys still have the metallic (non-directional) bonding and structure of pure metals
but are harder than the individual pure metals they are made of.
o
By adding a second element into the metal structure, there is a major effect on the
metal ions ability to move past each other. Unlike the pure metal, the alloy is made
of different types of atoms and therefore different sized atoms arrange in layers. The
layers of atoms cannot slide over each other as easily, so the structure is much
harder and can take a larger weight load before the layers of ions are forced to
move.
See the diagram below:
In the end, the property changes enhance metal properties. We use metals for a variety of
properties; alloying them makes them even better suited for their specific uses.
There are many examples of alloys used in our everyday lives. Some examples include:
o Steel which is an alloy of iron and carbon. Used in buildings and many other
structures, there are a lot of different steel alloys depending on the ratio of iron to
carbon.
o Amalgam which is an alloy of mercury, silver and other metals used in dental fillings.
o
Brass which is an alloy of copper and zinc used in locks, gears, and valves. Brass is
resistant to rust and corrosion, so moving parts made of brass will not damage as
quickly due to moisture or wear and tear. Brass is also used in musical instruments.
Smart alloys are alloys which are able to return to their original shape after being bent, by
heating or electrifying it. This is a useful property for making glasses frames and other items that
need to be a little bit flexible.
Topic Notes
In this lesson, we will learn:
The definitions of polymer, monomer and polymerization.
To describe the uses of some polymers with examples.
How to identify the repeating unit and monomer unit when studying a polymer chain.
Notes:
Polymers are large molecules made of many repeating smaller units chemically joined
together. The word polymer shows this; it has two roots, "poly" meaning many, and "mer"
meaning molecule. Most are man-made, but some well-known natural materials are polymers –
wool, silk and even DNA in our bodies can be called a polymer!
The repeating small molecule units that make up the polymer are called monomer units. This
word has roots "mono" meaning one, so monomer means "one molecule" where polymer means
"many molecules". The most common monomers that make polymers are alkenes, which are
mostly sourced from crude oil.
The most common polymers in our daily lives are man-made and the monomers are alkenes,
which have a carbon-carbon double bond. These join together in a chemical process
called polymerization, where many small monomers join to make a large combined polymer
chain(s).
Polymers are extremely useful in our daily lives – most plastics are polymers, and their name
often begins with poly-. Some include:
Polyethene (AKA polythene) which is a polymer made of ethene monomer units.
Polyethene is used in plastic shopping bags and drinks bottles.
Polystyrene which is used in packaging materials
Polychloroethene (AKA polyvinyl chloride; PVC) is used in piping and for electrical
wire insulation.
Identifying polymer molecules is about identifying repeating parts of a larger molecule. See the
examples below for identifying and showing polymers, their repeating unit, the monomer
structure and their relation to one another:
Topic Notes
In this lesson, we will learn:
To recall the definition of allotrope and name some carbon allotropes.
How the bonding of a material leads to its specific 3d-structure.
How the 3d structure of a material explains its unique properties using carbon allotropes as an
example.
Notes:
The carbon atom can make four covalent bonds to other atoms to fill its outer electron shell.
Partly because of this high number, it has multiple forms or allotropes when samples of carbon
are found in nature. An allotrope is the unique bonding arrangement and structure that atoms of
an element make.
There are three important examples of carbon allotropes: diamond, graphite, and Buckminster
Fullerene (and carbon nanotubes).
o
o
Diamond is an allotrope of carbon where each carbon atom is covalently bonded to
four other carbon atoms.
The four strong covalent bonds around each carbon atom makes
a tetrahedral shape around each atom.
This bonding creates a large lattice structure because every carbon
atom is connected to four others. Diamond is practically one giant
molecule because every single carbon atom is (eventually) connected
to all the other atoms in the lattice through this bonding. For this
reason, we say diamond has a giant covalent structure.
This structure gives diamond its unique properties:
Diamond is clear and colorless, as the carbon atoms in this
structure reflect visible light. This also makes it shiny and
lustrous which is why it is desirable in jewelry.
Diamond is extremely hard, as deforming diamond would
need you to deform the entire giant lattice that every
'diamond carbon' atom is part of. This makes it useful in
cutting tools and drills.
Diamond is insoluble in water, as interactions with the
water molecules are not nearly strong enough to 'pull
apart' the giant covalent lattice, so it does not dissolve.
Diamond cannot conduct electricity, as no free electrons
are found in the diamond lattice. All the electrons of all
carbon atoms in the lattice are locked up in covalent bonds
to each other, so no carrying of electric charge through the
lattice can take place.
Graphite is an allotrope of carbon where each carbon atom is covalently bonded to
three others in layers of 2d sheets.
The three strong covalent bonds on each carbon atom are equally
spaced in 2d 120^{o}120o apart from each other. There is one
electron on each carbon atom still unbonded or 'free'.
This bonding gives graphite a structure of layers of 2-dimensional
carbon atom sheets. These sheets stack on top of each other with weak
stabilising interactions due to the spare electron of each carbon atom.
This unique structure of graphite gives it its unique properties that are
quite different from diamond:
Graphite is a dark grey/black colour and is opaque as it
absorbs visible light that interacts with it.
Graphite is a smooth, slippery material because the
stabilising forces between the sheets of carbon atoms are
quite weak. This means applying some pressure to
graphite makes the layers slide over each other quite
easily. This is how pencils work: graphite layers slide off of
the pencil and onto the paper we write on when we press
the pencil down! It’s also used in lubricants, which are
chemicals that deliberately reduce friction.
o
Buckminster Fullerene is an allotrope of carbon where each carbon atom is bonded
to other carbons to make a 3d spherical ball of 60 carbon atoms known as a
'buckyball'.
Graphite is a good conductor of electricity,because each
carbon atom has a spare electron. The spare electrons of
all the carbon atoms are delocalised - they are capable of
moving and carrying electric charge throughout the sheet
that it is part of and through weak interactions that hold
the layers close together. For this reason, graphite is used
in electrodes for electrolysis experiments.
Buckminster Fullerene is one of a larger category called Fullerenes.
Some Fullerenes have tube-like structures which have a very large
surface area to volume ratio. These fullerenes are called nanotubes and
they have unique desirable properties such as conducting electricity
and high strength combined with lightness. Many are also useful
catalysts – the high surface area to volume ratio is a common property
seen in nanomaterials.
Carbon is just one example of an element which has multiple allotropes. We have only looked at
three here, but another is coal which is a very important fuel. The difference between coal,
graphite, diamond and Buckminster-Fullerene is simply how the carbon atoms are bonded and
arranged together, they are all 'types of carbon'. A summary of the allotropes and their features
are below:
Allotrope
Diamond
Graphite
BuckminsterFullerene / carbon
nanotubes
Structure
Giant covalent
tetrahedral structure
Giant covalent 2d
layered sheets
3d hollow sphere / 3d
hollow cylinders
Melting point
Very high
Very high
High
Conducts
electricity
No
Yes
Yes (nanotubes)
Hardness
Very high
Low
Uses
Cutting tools,
jewelry
Pencils, electrodes
Catalysts, medical
science.
The different carbon allotropes are a good example of the
bonding \to→ structure \to→ properties link in material chemistry:
o
The nature of bonding in a substance leads to the material's structure.
The bonding will tell us how the negatively-charged electrons are interacting with
the atoms which contain the positively-charged nucleus. This could lead to strong
electrostatic forces (large positive charges attracting large negative charges like in
ionic or metallic bonding), a giant covalent lattice, or a simple covalent molecule
with only weak forces keeping molecules close together.
The structure of a substance leads to explaining the properties that we
observe of the substance:
Can the particles in the structure move freely and interact
with electric charge? Charged particles will interact with
other charged particles. If the structure has charged
particles with free movement, they will be able to carry
electric charge and therefore conduct electricity. If there
are no charged particles or the particles are unable to
move throughout the structure, this won't be possible.
This might affect the hardness and strength of the material
too – if particles can/will move when a force is applied, the
general structure will change shape!
What forces of attraction are keeping the whole structure
together? Recall the particle model; in any substance the
solid state has particles packed together with 'low energy'
because the current energy is not enough to overcome the
attractive forces keeping the particles together.
In a gas state, particles are far apart with 'high energy' as
that high energy has overcome the attractive forces that
were holding them together - that's why the particles are
far apart and gases take up more volume than solids!
If those forces of attraction are strong then a lot of energy
will be needed to overcome them. These structures will
have a high melting/boiling point.
Topic Notes
In this lesson, we will learn:
To recall the definition of nanomaterials and nanoparticles.
How nanomaterials are different to regular materials and why they are of interest to scientists.
Examples of nanomaterial uses, potential benefits and risks associated with them.
Notes:
Nanotechnology is technology that makes use
of nanomaterials and nanoparticles. Nanomaterials are any materials that have a size
measurable in nanometres; one nanometre is one billionth of a metre (1x10-9 m). The practical
range is 1-100 nm.
Nanoparticles are interesting to scientists because a chemical substance can have different
properties depending on if it is a nanoparticle form or not. The most interesting general feature
of nanomaterials of any substance is a very high surface area to volume ratio, which generally
makes materials a lot more reactive.
o Surface area to volume ratio is a ratio of how much of the material (the atoms it's
made of) are exposed on the surface of the material – surface area - compared to
the total space the material takes up – the volume.
Because only the atoms on the surface (which are exposed to other substances!) of
a material can react, materials with a higher SA:V ratio are more reactive.
o
Remember that any material can technically be a nanomaterial – it just needs to be
made on a nanometre scale!
New nanomaterials are being used in a number of products such as:
o
o
o
Take two identical, equal sized cubes of a material. Leave one
unchanged, but cut the other in half vertically, horizontally and through
the middle. You will have 8 smaller cubes of the second sample, with
the same original amount/volume but a larger surface area because
more of the material is exposed to the surroundings now. More of this
material can now react with the surroundings at any time. See the
diagram below:
Sports equipment, to deliver strength whilst still being very light.
Sun creams that absorb UV radiation better.
The hollow structure of nanotubes means they could be used as 'capsules' to deliver
drugs and medicines (the contents of the capsules) into the body, especially to the
brain which could help research on brain-related conditions such as Parkinson's
disease or Alzheimer's.
Because a lot of the nanotechnology in use is quite new, a few concerns about its use have
arisen:
o
Because of the extremely small size of nanoparticles, there is a possible risk that the
particles will be able to pass the blood-brain barrier which could affect internal
biological processes.
o
There is a concern that because nanoparticle use is very recent, it is difficult to know
the long-term effects of exposure to nanoparticles. These findings may take years to
establish.
1. Understand how equilibrium and reversible reactions occur.
The reaction between substances A and B to produce C and D is described below in an
equation.
A (g) + 2B (g) \,→\, C (g) + D (g)
The reaction takes place at high temperature and pressure with the container sealed.
i.
ii.
Explain how sealing the reaction container could establish an equilibrium.
Explain why leaving this reaction unsealed creates other practical problems.
Topic Notes
In this lesson, we will learn:
The definition of reversible reaction and dynamic equilibrium.
How the open and closed state of a system affect equilibrium.
Notes:
We know chemical reactions as going from reactants to products, but many chemical reactions
can go from products 'back' to reactants. Reactions which can go 'both ways' are called reversible
reactions.
o
In reversible reactions there are terms given to the 'direction' of the reaction which will be used
in this chapter:
o
o
In the kinetics chapter, we learned about the activation energy barrier preventing
reactants from forming products in chemical reactions. For a chemical change to
occur, reactant particles need sufficient energy and correct orientation when
colliding. These are the conditions of a successful collision.
As long as the conditions for a successful collision are met, there is no reason why
the transformation cannot go in the other way too! All that is needed is a certain
activation energy.
The forward reaction is the chemical change from reactants to products with respect
to a given chemical reaction.
The reverse (aka backward) reaction is the reverse change from products to
reactants.
In many cases, reversible reactions do not seem to be reversible because they are performed in
open systems. Two more definitions for this chapter are below:
o
An open system is an environment where other substances or energy e.g. heat or
light can enter and leave.
o
A closed system is an environment where substances and/or energy cannot enter and
leave.
When a reaction takes place in an open system, the products escape or are
removed from the reaction vessel to proceed with their intended use. The
products are therefore removed from the conditions that could cause the
reverse reaction to occur, and without the products available, the system
will not be able to make the reverse reaction happen!
When a reaction takes place in a closed system, the products of the desired
forward reaction cannot escape. This is often done when the desired
products are gases so they are trapped in the reaction vessel and won't be
lost. However, the products of the forward reaction are the reactants of the
backward reaction – so this can start occurring!
Under some conditions, the rate of the reverse reaction equals the rate of the forward reaction,
creating a balanced system of constant change. This is called dynamic equilibrium. This
sometimes creates the appearance that the reaction has "stopped" but it has not – it is simply
making products as quickly as it is re-making reactants, so the amounts of each do not change!
o An analogy of this effect is filling a swimming pool which has a hole in it that is
leaking water. If the pool is being filled by a hose at the same rate it's being drained
by the hole, it is at equilibrium – constantly changing in both ways at the same rate!
1. What happens at equilibrium?
2. Recall dynamic equilibrium.
3. What happens when changing conditions at equilibrium?
4. Explaining changes to equilibrium.
0/1
Examples
Lessons
1. Apply Le Chatelier's principle to predict changes in equilibrium position.
The reaction equation below shows an EXOTHERMIC reaction at equilibrium:
2A(g) + B(g) \, \rightleftharpoons \,⇌ C(g) + D(g) \quad \triangle H△H = -89 kJ mol-1
Predict and explain the change in equilibrium position with the following changes of
conditions happening separately:
i.
ii.
iii.
An increase in pressure.
A decrease in temperature.
Addition of a catalyst.
Topic Notes
In this lesson, we will learn:
To recall Le Chatelier's principle when studying equilibria.
How to predict changes to equilibria given changes in reaction conditions.
How to explain changes in equilibrium position due to changes in reaction conditions.
Continuing from Dynamic Equilibrium, when more chemical reactions were studied, and more
equilibria were found, chemists started changing the conditions (such as temperature and
pressure) of the reaction to see what this did to the equilibrium they saw.
This led to them finding Le Chatelier's principle:
o When a reaction at equilibrium is disturbed by a change in conditions, the system
will respond in a way that COUNTERACTS the disturbance and then re-establish a
new equilibrium.
Notes:
The system 'counteracts the disturbance' by favoring the forward or the backward reaction; it will
do the opposite of whatever effect the disturbance had on the reaction! 'Favoring' a reaction
means that reaction rate increases (causing a change in the ratio of reactants to products in the
system), before a new equilibrium is reached as the two rates become equal again. Once this
happens, the new ratio of reactant to product concentration won’t change unless equilibrium is
disturbed again - in this case, the above happens again.
o Remember, 'at equilibrium' only tells us that the forward and reverse reactions are
happening at the same rate. Equilibrium does not tell us how much product and
reactant there is in the system!
When a reaction at equilibrium is disturbed, it will work to re-establish equilibrium under these
new conditions. If some facts about the reaction are known, the change to equilibrium position
can be predicted from a change in conditions:
o If temperature is increased:
In an exothermic reaction: The system will respond to favor the reverse
reaction which leads to a greater concentration of reactants being
observed in the reaction. An exothermic forward reaction, by definition,
causes a net release of heat energy to the surroundings. The system
therefore counteracts the disturbance of the temperature increase by
temporarily favoring the reverse reaction, which will be the
endothermic opposite of the exothermic forward reaction. This
endothermic reverse reaction has a net absorbing of heat effect,
absorbing the increase in temperature that initially disturbed the
system. This is how Le Chatelier's principle works! When the reverse
reaction is favored, we say the equilibrium has shifted to the left as the
left-hand side of the reaction equation shows the reactants.
In an endothermic reaction: The system will respond by favoring the
forward reaction. This is for the same reasons as above; the system will
favor the endothermic forward reaction, which absorbs heat and
counteracts the increase in temperature that it was disturbed by.
Favoring the forward reaction means the equilibrium shifts to the
right. In this case, more products will be produced in the system.
o If temperature is decreased:
In an exothermic reaction: The system will respond to favor the forward
reaction. This is because the exothermic forward reaction has a net
releasing of heat effect, which will raise the temperature and
counteract the decrease in temperature that initially disturbed it. In this
case we say the equilibrium has shifted to the right and more products
will be produced.
In an endothermic reaction: The system will respond to favor the
reverse reaction. This is because the reverse reaction will be
exothermic, causing a net release of heat energy to the system, raising
the temperature that initially disturbed it. The equilibrium will shift to
the left and more reactants will be present in the system.
o
If pressure is increased, count the number of moles of gas on both sides of the
reaction equation:
o
o
When there are more moles of gas in the products than the reactants,
the system will respond to favor the reverse reaction and the
equilibrium will shift left. This has the effect of 'cutting back' the
number of gas particles in the system as there are more of them in the
products than the reactants. This leads to less gas particle collisions
which decreases pressure, which counteracts the initial disturbance of a
rise in pressure!
When there are less moles of gas in the products than the reactants,
the system will respond to favor the forward reaction and the
equilibrium will shift right. This counteracts the disturbance in the same
way as explained above.
If pressure is decreased, count the number of moles of gas on both sides of the
reaction equation:
When there are more moles of gas in the products than the reactants,
the system will respond to favor the forward reaction and the
equilibrium will shift right This has the effect of increasing the number
of gas particles in the system as there are more gas moles in the
products than the reactants. This leads to more gas particle collisions
which increases pressure, counteracting the initial disturbance of a
pressure decrease!
When there are less moles of gas in the products than the reactants,
the system will favor the reverse reaction and the equilibrium will shift
left. This leads to an increase in the number of gas particles in the
system because there are more gas moles in the reactants than the
products. This counteracts the initial disturbance of the pressure
decrease.
If a catalyst is added to the reaction mixture, there is no change to the position of
equilibrium. A catalyst does not change equilibrium; it simply allows equilibrium to
be reached quicker.
The table below summarizes the changes to equilibrium position caused by a change in
conditions:
Even though chemists know how to change a reaction at equilibrium to make the largest amount
of product, there are practical issues with shifting the equilibrium.
o Increasing the pressure on a closed system can be very expensive and there are
safety considerations at very high pressure.
o Many reactions, such as the Haber process, have an exothermic forward reaction.
This strangely means that to shift the equilibrium to make more product, you need
to cool the reaction down which will reduce reaction rate.
Because of these and other factors, compromise conditions are often used in establishing the
ideal conditions for a reaction at equilibrium.
1. What is the equilibrium constant?
2. The equilibrium constant and equilibrium expression.
3. Changing the equilibrium CONSTANT.
4. Keq: a number for "where is the equilibrium?"
5. Heterogeneous systems and equilibria.
6. Reaction quotient, Q.
0/6
Examples
Lessons
1. Write the expression for the equilibrium constant, Keq and interpret its value.
The equation for the decomposition of compound A, is below:
A (g) \, \rightleftharpoons \,⇌ 2B (g) + C (g)
At 298 K, Keq = 4.5*1015
a. Write an expression for Keq for this reaction.
b. What does the value of Keq at 298K tell you about the reaction mixture?
2. Calculate the equilibrium constant for the reaction at equilibrium.
The decomposition of PCl5 is shown by the equation below:
PCl5 (g) \, \rightleftharpoons \,⇌ PCl3 (g) + Cl2 (g)
This reaction was started at room temperature (298K) by placing 0.5 mol of PCl5 in a 20 L
container. When the reaction came to equilibrium, 0.3 mol of Cl2 (g) was detected.
a. Find the number of moles of each substance in the equilibrium mixture.
b. Calculate the equilibrium constant, Keq given the equilibrium quantities
found in question a).
c.
Using the previous Keq equilibrium constant, what would be the equilibrium
amounts of PCl3 and Cl2 if 0.8 mol of PCl5 was present?
d. This equilibrium mixture is then heated up to 400K. Explain whether or not,
and if so how, this will affect the Keq value.
3. Calculate the reaction quotient Q and predict changes to the reaction conditions based on
Le Chatelier's principle
a. consider the reaction:
N2 (g) + 3H2 (g)\, \rightleftharpoons \,⇌ 2NH3 (g)
If the reaction above is said to have a Keq = 0.180 at a given temperature
and current partial pressures at this temperature in the vessel are
o
o
o
N2 = 0.6 atm
H2 = 0.6 atm
NH3 = 0.2 atm
What direction is the reaction currently going to favour?
Topic Notes
In this lesson, we will learn:
To write an expression for the equilibrium constant Keq.
How to interpret the value of Keq and describe the reaction using this value.
How to use the equilibrium expression with equilibrium concentrations to solve for Keq (and vice
versa).
To write an expression for the reaction quotient, Q, and learn the difference between Q and Keq.
We now know the definition of equilibrium; a chemical process where the forward reaction rate
is equal to the reverse rate. Be careful – this tells us nothing about how much product or reactant
is there! To find that, we need to use the equilibrium constant expression.
Using measurements of reactant and product concentrations, it is possible to find what is called
the equilibrium constant, Keq, (sometimes Kc) of a given reaction at equilibrium. This is done using
the expression:
For the reaction at equilibrium:
Notes:
aA + bB \rightleftharpoons cC + dDaA+bB⇌cC+dD
Keq is calculated by:
\large K_{eq} = \frac{[C]^c[D]^d}{[A]^a[B]^b}Keq=[A]a[B]b[C]c[D]d
Be clear with your language:
o
o
o
The whole equation is the equilibrium expression.
Keq is the equilibrium constant.
Keq is the general term – if the equilibrium is measuring concentration (in mol dm-3) it
might be called Kc. Kp would be used if it was partial pressures (for gases).
The equilibrium constant is called a constant because it is not affected by changes
in some conditions. Changes to concentration of reactants or products and changes in pressure
do not affect the equilibrium constant!
o Remember Le Chatelier’s principle: the system will counteract any change made. If
you add reactant, more product will be made to counteract the change. This keeps
Keq constant in the long run.
o
Changing temperature WILL affect Keq, depending on whether the reaction is
endothermic or exothermic.
Because of this, ALWAYS quote Keq with a temperature.
The equilibrium expression looks complicated, so breaking it down using simple math can help.
o It is a fraction. It has terms for amount of product and reactant.
o
You can write fractions as ratios. So…
o
It is a ratio of products to reactants in the reaction. Written as a decimal, the value
of Keq tells us something important:
Keq is smaller than 1: There is less product than reactant in the reaction
mixture. The smaller the value of Keq, the less product there is.
Keq is approximately 1: There is roughly the same amount of product as
reactant in the reaction mixture.
Keq is larger than 1: There is more product than reactant in the reaction
mixture. The larger Keq is, the more product compared to reactant.
When writing Keq for heterogeneous systems, where the substances are not all in the same phase,
ignore any substances in the solid state. Solid reagents do not affect the equilibrium constant;
everything else in the Keq expression is written as normal.
For example, let’s look at the thermal decomposition of calcium carbonate:
CaCO3 (s) \, \rightleftharpoons \,⇌ CaO (s) + CO2 (g)
If CO2 escapes the reaction vessel because it’s open, equilibrium cannot and will not be
established. CO2 alone dictates whether the equilibrium happens, so that is all the Keq expression
contains.
Keq = [ CO2 (g)]
Remember that Keq is only used for reactions at equilibrium.
The reaction quotient (symbol Q) is used for reactions not at equilibrium to reveal which
direction a reaction favours. Practically, Q is a Keq value for reactions that are not yet at
equilibrium!
It is calculated using largely the same information as Keq would be.
For the reaction:
aA + bB \,→\, cC + dD
We find Q by calculating:
Q =Q= \large \frac{[C]^{c} [D]^{d}} {[A]^{a} [B]^{b} }[A]a[B]b[C]c[D]d
This would be for reactants and products in the aqueous or gaseous phase, where concentration
is measured by partial pressure (see Kp and partial pressure). Like with Keq, pure solids and liquids
are not included in this calculation.
For some reactions, Q isn’t very useful. Reactions that go to completion will have an infinitely
large Q (all product, no reactant), and reactions that don’t proceed will have a Q value equal to
zero. A reaction with Q = 1 is already at equilibrium.
But many aqueous and gaseous reactions can go to equilibrium. Recall Le Chatelier’s principle: Q
is useful because comparing Q to Keq lets us predict changes to concentration as the reaction tries
to reach equilibrium.
You can think of Q then as a pendulum; Keq is the point at rest and Q is where it currently is:
o
If Q is smaller than Keqfor a reaction, the products will be favoured. Q > Keq suggests
there are more reactants in the mixture than the ideal equilibrium concentrations,
so according to Le Chatelier’s principle there will be a shift back toward the
products.
o
If Q is equal to Keq, there is no favouring of products or reactants. Q = Keq means the
reaction is already at ideal equilibrium concentrations. In this situation, nothing
would be expected to change.
If Q is greater than Keq, the reactants will be favoured. Q < Keq suggests that there is
more product in the vessel than the ideal equilibrium concentration, so the
equilibrium will shift back toward the reactants to remove this product surplus.
o
This Q value is larger than the quoted Keq. Which means there is currently more product than
there is at ideal equilibrium conditions. Applying Le Chatelier’s principle, we expect that in the
current conditions, the reaction will favour the reactants
1. Introduction to the Periodic Table
2. Beginning: Trying to organize elements.
3. Dobereiner: Grouping elements
4. Newlands: Periodic patterns in elements
5. Mendeleev: Predicting elements.
0/1
Examples
Lessons
1. Recall the contributions of scientists that helped develop the Periodic Table.
i) Which scientist was the first to make a breakthrough in organising elements by their
properties?
a) Johan Dobereiner
b) John Newlands
c) Dmitri Mendeleev
ii) Which scientist was the first to make a breakthrough in organising elements in repeating
(periodic) patterns?
a) Johan Dobereiner
b) John Newlands
c) Dmitri Mendeleev
iii) Which scientist was the first to make a breakthrough in predicting the properties of
undiscovered elements?
a) Johan Dobereiner
b) John Newlands
c) Dmitri Mendeleev
Topic Notes
In this lesson, we will learn:
• The contributions of key scientists which led to the modern Periodic Table.
• An example of how a scientific theory relies on measurement and a standardized method.
• The key features of a scientific theory and its emphasis on empirical observation and prediction.
Notes:
Like the development of atomic theory, developing the Periodic Table has taken time and
contributions by many scientists, each with their own theories and experiments, to lead to its
current state today.
The early work built on John Dalton's work, which tried to identify elements by their unique mass
because this was the most obvious property scientists could measure. Because quality of
equipment and analytical methods were poor and there was no standardized, 'proper' way to
measure atomic mass, there were inconsistencies in different scientists' measuring the mass of
elements. This held back progress; to organize, scientists measuring events or objects need
consistency to spot any patterns emerging.
The first move toward anything resembling the current Periodic Table was by Johan Dobereiner.
He showed that the appearance and the reactions of certain known elements were quite similar.
Because these certain elements with similarities came in threes, he called these groups triads.
Some of his triads (A triad of Li, Na, K and a triad of Cl, Br and I) survived and now form groups in
the current periodic table!
In the 1860s, John Newlands showed by ordering the elements by mass, with hydrogen first,
every eighth element had similarities in its properties. He called this the law of octaves. This was
seen as a breakthrough in the arrangement of the elements – it was the beginning of the term
'periodic' being used to describe the elements, meaning a repeating pattern. However, his
ordering had inconsistencies, such as placing metals in an octave with non-metals. This led to
the suspected existence of undiscovered elements.
In 1869, Dmitri Mendeleev published his work where he organized the elements according to
properties and their masses. It received very little attention to begin with, but it was noticed
after being republished. Mendeleev also noticed that when elements were ordered by mass,
there was a periodic (repeating) pattern of chemical properties. The genius in Mendeleev's work
was in doing the following:
o He organized the elements by row (called a period) and by column (called a group),
where the groups showed the elements that had common properties.
o He chose to move some elements around the table, prioritizing grouping elements
by their common properties, not ordering by their mass.
o
He deliberately left gaps in his table where he supposed the existence of
undiscovered elements. He even suggested the properties of these elements, using
the properties of the groups in his table. When discovered, they matched.
Mendeleev's table forms the basis of the current Periodic Table. The only major exception to his
work – the existence of the noble gases – slotted in at the end of the table (group 0) when
discovered. This didn't affect the pattern in his table.
Mendeleev's Periodic Table is a classic example of a successful scientific theory. For any theory to
be scientific, it needs to:
o
o
o
o
Be able to explain the current empirical evidence. Does it explain why we see what
we see in experiments and in the observable world?
Be testable by future experiments. If a theory and its supporters want to be proved
right, we need to be able to plan and do experiments that prove it at all!
Be able to explain new evidence when it appears. Scientific theories need to account
for all evidence; they can't 'pick and choose' when to work.
If a theory can't explain new evidence, it needs to be either revised or replaced by
another theory that is also testable and does explains the evidence so far!
1. Using the Periodic Table
2. Changing Mendeleev's table.
3. Basic structure and layout of the table.
4. Blocks, groups and shape of the modern Periodic Table.
5. Why does the periodic table have subshells or 'blocks'?
0/4
Examples
Lessons
1. Apply knowledge of the periodic table structure to classify elements.
For each chemical element, state the chemical group it is in, whether it is a metal or non-metal, and
the block of the periodic table in which it belongs.
a. i) Ga
ii) Pd
iii) U
b. i) Cl
ii) Xe
iii) Ba
2. Apply your knowledge of the structure of the periodic table to identify elements of similar
properties.
For each chemical element, state another chemical element which shares similar properties to it.
a. i) Ru
ii) Li
b. i) C
ii) Br
Topic Notes
In this lesson, we will learn:
The changes made to the Periodic Table since Mendeleev's major contribution.
The basic structure and layout of the modern Periodic Table.
The precise grouping and arrangement of the Periodic Table.
As seen in the lesson on History and Development of the Periodic Table, one of the main reasons
the development of the Periodic Table was initially slow is because data quality was often poor.
Inaccurate measurements meant organizing elements correctly was hard.
As improvements meant more and better data could be used, some changes were made to
Mendeleev's Periodic Table. One big change was in ordering the table by atomic number (proton
number) rather than by atomic mass. This solved the problem of isotopes; when arranged by
atomic mass, some elements look like they are in the wrong place in the table (e.g. K has a lower
atomic mass than Ar, even though K has one more proton). The current periodic table, then,
obeys the Periodic Law: The properties of the elements repeat periodically when ordered
according to their atomic numbers.
The basic layout of the current periodic table has:
o Columns known as groups, the elements inside each of which having similar
properties.
o Rows known as periods. The elements in a given period have the same number of
electron shells.
The current periodic table is arranged in a way that separates metals and non-metals:
o Metals are found on the left hand side of the periodic table.
o Non-metals are found on the right hand side of the periodic table.
o Metalloids or semiconductors are found on the border between metals and nonmetals. These elements have a mix of metal and non-metal properties.
o About 80% of the elements in the periodic table are metals. The 'border' between
metals and non-metals begins with boron and runs diagonally down to between
non-metal Astatine (At) and Polonium metal (Po).
Notes:
The current periodic table has its specific shape to clearly show the different "blocks" of
elements, in terms of their electron subshells (see lesson Electronic structure: Subshells).
o
o
o
o
The first two groups (columns) form the s-block, the taller section on the left. This
contains the alkali metals (group 1) and the alkali earth metals (group 2).
The next ten groups form the d-block, generally known as the transition metals. This
is the central block where the table 'dips'.
The next six groups, where the table rises up again, is the p-block. It contains the
metalloids and non-metals, including the halogens and the noble gases.
The 'island' normally shown alone at the bottom of the periodic table is the f-block.
It is actually an insert, inside the lower part of the d-block. It contains two rows
known as the lanthanides and actinides.
The periodic table has its specific shape to indicate the electron sub-shells and the block which a
given element falls into. This is based on experimental data; the energy cost of removing the first
outer shell electron (the ionization energy) from a sample of a given element follows a consistent
pattern across the period (see lesson Periodic Trends: Ionization energy). This pattern led
chemists to develop the sub-shell theory where not all electrons in a shell are in the same state –
some electrons were in s orbitals, others p, d or f orbitals. The outer electrons occupying these
orbitals, as suggested by experimental data, is represented in the shape of the periodic table we
use.
1. Metals and non-metals in the Periodic Table
2. The metal / non-metal trend.
3. Metal and non-metal properties.
4. Investigating elemental properties.
0/6
Examples
Lessons
1. Identify metals, non-metals and metalloids in the Periodic Table.
Based on its position in the periodic table, suggest whether the element is a metal, non-metal or
metalloid.
a. Pd
b. Si
c.
F
d. K
2. Identify metals, non-metals and metalloids by their physical properties.
Read the material properties below and determine if they are metal, non-metal or metalloid
properties.
a. A silvery-white, shiny solid which is a good conductor of electricity, and a dull yellow
solid which is a poor conductor of electricity
b. A brittle blue-white shiny solid, which is a fair conductor of electricity, and a pale
green gas, which is a poor conductor of electricity.
Topic Notes
In this lesson, we will learn:
The Periodic Table's arrangement of metals and non-metals
The range of metal and non-metal properties and the importance of variety in the properties of
elements.
How to classify elements as metal, non-metal or metalloids based on their properties.
As seen in lesson Structure of the periodic table , the current Periodic Table shows elements
arranged in terms of metals and non-metals. Metals are on the left whilst non-metals are found
on the right of the table.
This way of classifying elements is important because whether an element is a metal or not
suggests a lot of its chemical and physical properties.
In general, metal properties are as follows:
Notes:
o
Metals reflect light - they are shiny, not dull.
o
Metals conduct both heat and electricity well.
o
Metals have a high melting and boiling point and are solids at room temperature
(except mercury).
o
Metals are hard, but malleable (when heated, they can be hammered into desired
shape) and ductile (they can be drawn out to form wires).
In general, non-metals have properties opposite to metals:
o Non-metals are usually dull and don't reflect light.
o
Non-metals are poor conductors of both heat and electricity.
o
Non-metals have a relatively low melting and boiling point – many are gases at room
temperature.
o
If a non-metal is a solid at room temperature, it's normally brittle, meaning its shape
cannot easily be changed or manipulated like a metal can.
The elements in the periodic table show a spectrum of metal character. This means there is not
always completely metal or non-metal properties in an element – the most important examples
of these are the metalloids, or semiconductors. These elements on the border of metals and
non-metals display a mix of metal and non-metal properties.
The general trend in the periodic table shows increasing metallic character going from right to
left in the table, and going down the table toward the bottom. "Increasing metallic character"
means the elements will have more metal-like properties.
Knowing the spectrum and variety of properties that different elements have is important for
chemists to help choose the right material for the right use or task.
1. Chemical "Forces of attraction"
2. Principles of electrostatic forces.
3. Using the principles to explain periodic trends.
4. Trend in melting point across a period.
0/2
Examples
Lessons
1. Explain trends in atomic radius down a group.
Explain the trend in atomic radius observed in group 1 of the Periodic Table.
2. Explain trends in atomic radius across a period.
Explain the trend in atomic radius observed going across Period 2 of the Periodic Table.
Topic Notes
In this lesson, we will learn:
To understand the principles of electrostatic forces and how they are used to explain
experimental data.
To explain trends in atomic radius down a group using principles of electrostatic forces.
To explain trends in atomic radius across a period using principles of electrostatic forces.
To understand and explain the trend in melting and boiling points of elements across a period.
Notes:
Chemists have found, through experimenting, some principles of electrostatic forces – forces that
exist because charged particles attract or repel each other. The principles are:
o #1: Oppositely charged particles attract each other, while particles of like charge
repel each other.
o #2: The greater the charge difference of two particles, the greater their force of
attraction (for example, the attractive force between a 2+ ion and a 2- ion is stronger
than the attractive force between a 1+ ion and a 1- ion).
o #3: Attractive forces between oppositely charge particles decrease with distance.
o
#4: Repulsive forces between like-charged particles decrease with distance.
These principles form a theory that helps explain the trends that chemists see in their
experimental data, such as in the change in atomic radius and first ionization energies of the
elements
Atomic radius measures the distance between the nucleus and the outermost electron(s). There
is a clear trend in atomic radius when going down the elements in a group or moving across
elements in a period. Using the principles of electrostatic forces, we can explain both trends.
Going down a group of elements:
o Each element further down the group has an extra inner shell of negatively charged
electrons between the outermost electrons and the positively charged nucleus.
o These negative inner electron shells are also attracted to the positive nucleus (see
#1 above), and are ‘shielding’ the positive charge of the nucleus from the outermost
electron shell. This offsets the extra positive charge from the extra protons in the
nucleus.
o
In effect, going down a group, the atomic radius is determined by the number of
inner electron shells between the nucleus and the outer electron shell.
o These extra inner electron shells repel the outer electron shell (see #1) as both are
negatively charged. Being too close to the inner electron shells would cause
repulsion (see #4). To reduce this, the outer shell is pushed further away from the
nucleus due to the repulsion and so it is less attracted to the nucleus (see #3 above).
This leads to larger atomic radius going down the group.
Going across a period of elements:
o
o
Each element further across the period has an extra proton in its nucleus,
strengthening its positive nuclear charge, and an extra negative electron in its outer
shell which is attracted to the nucleus (see #1).
This extra positive nuclear charge and extra negative charge of the outer shell
electrons leads to a greater force of attraction (see rule #2) and this effect is
stronger than the repulsion (see #1) of adding one extra electron to the outer shell
of electrons. This causes the outer electrons to be drawn in closer to the nucleus.
Because going across a period does not add extra electron shells, there is no extra
effect of electron shielding.
Melting and boiling points across a period also change across a period for a similar reason to the
change in atomic radius
o From Na through to Al, the elements have a giant metallic structure. This is a giant
lattice made of positive metal ions surrounded by an attractive force of delocalized
electrons – we call this metallic bonding.
o Going from left to right, the metal ions of the lattice are increasingly
positive (Na+ \enspace→\enspace Mg2+ \enspace→\enspace Al3+) and they
each attract more moles of electrons per ion:
o
Na \enspace→\enspace Na+ and one mole of electrons in the lattice
Mg \enspace→\enspace Mg2+ and two moles of electrons in the
lattice
Al \enspace→\enspace Al3+ and three moles of electrons.
This creates stronger metallic bonding by principle #2 above – a greater charge
difference between positive metal ions and the moles of electrons holding the
structure together. This explains the melting/boiling points in Al being substantially
higher than Mg, which is higher than Na.
1. Looking at Periodic Trends
2. Recap of Electrostatic principles
3. Definition of ionization energy.
4. The trend in ionization energy across a period.
5. Why are there anomalies in the ionization energies across a period?
0/1
Examples
Lessons
1. Recall and explain the trends in ionization energies across the periodic table.
Explain the trend in ionization energies across Period 2 of the Periodic Table, including any
anomalies.
Topic Notes
In this lesson, we will learn:
The definition of ionization energy and understand its significance to studying the elements.
To explain the trend in ionization energy by applying principles of electrostatic forces.
The anomalies in the ionization energy data to help develop understanding of electron shells.
Notes:
As seen in Periodic trends: Atomic radius, chemists have found, through experimenting,
some principles of electrostatic forces – forces that exist because charged particles attract or
repel each other. The principles are:
o
o
o
o
#1: Oppositely charged particles attract each other, while particles of like charge
repel each other.
#2: The greater the charge difference of two particles, the greater their force of
attraction (for example, the attractive force between a 2+ ion and a 2- ion is stronger
than the attractive force between a 1+ ion and a 1- ion).
#3: Attractive forces between oppositely charge particles decrease with distance.
#4: Repulsive forces between like charged particles decrease with distance.
Together the principles form a theory that explains what chemists see in the data of their
experiments, such as the atomic radius of chemical elements and their 1st ionization energies.
As seen in this chapter so far, arranging the elements by their proton number shows a number of
trends in the properties of the elements. This is true going down the table or “going down the
group”, and going across the table or “across the period”. The fact that these patterns repeat
themselves – they are periodic – is why the table of elements is called the periodic table of
elements!
Ionization energy is defined as the energy required to remove one mole of electrons from one
mole of gaseous atoms to form a positive ion.
More specifically: The first ionization energy is the energy required to remove one mole of the
most weakly-held electrons from one mole of gaseous atoms to form one mole of gaseous ions
with a single positive charge.
The successive ionization energies follow from the first: it is the energy required to remove one
mole of the next most weakly-held electrons from one mole of gaseous ions to form gaseous ions
with a one-greater positive charge. For example, the second ionization energy would be the
energy required to remove one mole of the most weakly-held electrons from one mole of 1+
charged gaseous ions, forming one mole of 2+ charged gaseous ions.
The 1st ionization energies of the elements show a very distinct pattern in the periodic table. For
chemists, it is very revealing to study the ionization energies in elements across a period because
it shows how difficult it is to remove one extra electron from the same outer electron shell!
As briefly talked about in Structure of the Periodic Table, the distinct shape of the periodic table,
where the s, p, d, and f blocks exist, is because of ionization energies.
The trend in ionization energy across a period (for example, period 2) is explained using
electrostatic forces:
o As you go across the period from left to right, each element contains one extra
proton in the nucleus, increasing its charge.
o Each further element also has one extra electron in its outer shell. This greater
charge difference between the positively charged nucleus and negative outer shell
electrons results in greater force of attraction (see principle #2) and the electrons
being attracted (principle #1) more strongly.
o
This means extra energy is required to be put in to overcome the force of attraction
and remove an outer shell electron - in general then, moving to the right of a period,
first ionization energy increases.
o
There is an anomaly in this trend for boron: boron's outer shell electron
configuration is 2s2 2p1 - it has one electron in the 2p subshell, which is
being shielded from the nucleus by the 2s subshell that is already full,
causing repulsion (principle #1), while the 2p orbital is further away from the
positive nucleus to begin with so is less strongly attracted to it (principle #4). This
effect overcomes the greater charge difference from an extra electron and proton. It
therefore costs less energy to remove the first electron from boron's outer shell
than the general trend would suggest.
o
There is another anomaly in this trend for oxygen: oxygen's outer shell electron
configuration is 2s2 2p4, where one of the 2p orbitals is now full with two electrons
paired for the first time (until oxygen, the electrons fill up one p orbital by
themselves, see Hund's rule). This increases repulsion (see principle #1) and
overrides the effect of greater charge difference attracting the electrons to the more
highly charged nucleus. This means less energy is required to remove the first
electron from oxygen's outer shell than the general trend suggests.
The ionization energy trend occurs in the 3rd period too. After each noble gas, there is a massive
drop in ionization energy e.g. from Ne to Na. These data helped developed understanding of
electron shells and subshells and the number of electrons they can hold:
o Using electrostatic principles (more negative electrons being attracted to a more
positively charged nucleus), we would expect greater attraction of the electrons by
the nucleus, and even more energy needed to remove (one mole of) electrons. So
what do the repeating – or periodic - drop in ionization energy mean?
o Our current theory says that the extra electron in boron must be in a different ‘state’
or sub shell than the last electron in beryllium. Why else would it cost a lot less
energy than beryllium to remove an electron?
o The idea of electrons being in shells and subshells was developed by quantum
mechanics, which also established the number of electrons the subshells could hold.
The trend in ionization energies practically shows you how easily an atom can form a positive
ion – by losing an electron, a positive ion is formed. Based on this, we can observe that it is
easier for metals to lose electrons and form positive ions than non-metals. This is related to
the electronegativity of an atom – the focus of the next lesson!
1. The periodic trends of electronegativity
2. Definition of electronegativity.
3. Trends in electronegativity in the Periodic Table.
4. Electronegativity and bonding.
5. Trends in metallic character across a period.
6. Trends in acid-base properties of oxide compounds.
0/2
Examples
Lessons
1. Recall and explain the trends in electronegativity in the periodic table.
Describe and explain the trend in electronegativity across the elements in period 2 of the
Periodic Table.
2. Recall and explain the trends in electronegativity in the periodic table.
Describe and explain the trend in electronegativity in group 2 of the Periodic Table.
Topic Notes
In this lesson, we will learn:
The definition of electronegativity and how it is measured.
To apply our understanding of electrostatic principles to the periodic trends in electronegativity.
To predict the electronegativity of elements compared to each other.
How metallic and acid-base properties change across a period.
Notes:
As seen in Periodic trends: Atomic radius, chemists have found, through experimenting,
some principles of electrostatic forces forces that exist because charged particles attract or repel
each other. Some principles are:
o #1: Oppositely charged particles attract each other, while particles of like charge
repel each other.
o #2: The greater the charge difference of two particles, the greater their force of
attraction (for example, the attractive force between a 2+ ion and a 2- ion is stronger
than the attractive force between a 1+ and a 1- ion).
o #3: Attractive forces between oppositely charge particles decrease with distance.
o #4: Repulsive forces between like-charged particles decrease with distance.
Electronegativity is the ability of an atom (specifically the nucleus) to attract bonding electrons to
its outer electron shell. It is measured using the Pauling scale fluorine is highest at 4.0 on the
scale, the most electronegative element, whilst francium is the lowest at 0.7 and is the least
electronegative element.
Electrostatic theory explains the trend in electronegativity in the Periodic Table, in the Periodic
Table both across a period and down a group:
o
As you go across the elements in a period, each element has an increased effective
nuclear charge, or Zeff attracting its outer shell electrons. Effective nuclear charge is
the positive charge that the outer shell electrons effectively feel from the nucleus.
To find this, subtract the number of inner electrons from the number of protons in
the atom.
In lithium for example, Zeff = 3 - 2 = +1.
This equation means that two of lithiums 3 protons are cancelled out
by the two inner shell electrons and only one is available for the one
outer shell electron. Using electrostatic theory: Li is a Zeff 1+ nucleus
attracting 1- of electron charge.
Moving across to beryllium, Zeff = 4 - 2 = 2+.
Using electrostatic theory: Be is a 2+ Zeff nucleus attracting 2 outer shell
electrons or a 2- charge..
Increasing Zeff means the nucleus attracts and holds its outer shell electrons with
increasingly greater force. It also better attracts other electrons to complete the
outer shell.
Therefore, as you go across the period, it is easier for atoms to attract bonding
electrons into their outer shell. This means electronegativity is higher.
o
As you go down a group of the periodic table, each element has an extra inner
electron shell as well as increased nuclear charge. However, the increased nuclear
charge is cancelled out by the extra inner shell, so Zeff is unchanged.
The extra inner shell of electrons also causes shielding of the nucleus. This is where
the outer electrons are repelled away from the nucleus because of the like-charge
inner shell electrons. In other words, positive attracts negative but not if there is a
big wave of negative charge between them!
This means that going down the group, nuclei lose their ability to attract and hold
onto bonding electrons. As a result, electronegativity decreases as you go down a
group in the Periodic Table.
o
Remember: Noble gases are not given an electronegativity value because their
atoms generally do not form bonds and since they already have full outer shells,
they do not attract electrons to complete full outer shells!
The trends in electronegativity mean fluorine is the most electronegative element. The effect
of electron shielding down a group is more influential than the effect of increased nuclear charge
across a period, so oxygen is the second most electronegative element (around 3.5 on the
Pauling scale), followed by chlorine (around 3.0).
The difference in electronegativity of atoms affects how different atoms bond with one another
and can lead to substances of varying properties. This is a very important part of chemistry which
the next chapter will look at – bonding between atoms and the properties of compounds they
make as a result!>.
Electronegativity affects the metallic character of an element (metals have very low
electronegativity) and as such, metallic character across a period changes. From left to right
across a period, metallic character decreases. For example, in period 3:
o
o
Sodium, magnesium and aluminium are metals;
Silicon is a metalloid (it displays properties of both metals and nonmetals);
o
Phosphorus, sulfur, chlorine and argon are all nonmetals.
There is a sliding scale of metallic character across the period which affects several properties.
One of these is the properties of oxide compounds. Using period 3 as an example:
o Sodium oxide (Na2O), magnesium oxide (MgO) and aluminium oxide (Al2O3) are
metal oxides with an ionic structure. As such, they usually have high melting points.
When dissolved in water, metal oxides form basic solutions with a pH of over 7. The
respective equations for sodium oxide and magnesium oxide reacting with water
are:
Na2O + H2O \,→\, 2NaOH
MgO + H2O \,→\, Mg(OH)2
Sodium hydroxide is a strong base, which is why sodium oxide in water creates a
basic solution. As you progress left to right, the metal oxides become less basic in
nature.
o
Aluminium oxide is amphoteric; it acts a base reacting with acids and acts as an acid
when reacting with bases, producing an aluminium salt both times. It also does not
react with water in the way magnesium and sodium oxide does. Its reaction with
acid is:
Al2O3 + 6HCl \,→\, 2AlCl3 + 3H2O
o
As silicon is a metalloid, silicon dioxide has very different properties to metal oxides
like Na2O or MgO, in fact it is very weakly acidic. SiO2 has a giant covalent structure
and does not react with water as the structure cannot be broken down by the
interactions with H2O molecules. As it is weakly acidic, it will react with some metal
oxides to produce silicon salts.
o
Phosphorus, sulfur and chlorine oxides are simple covalent molecules; comparing to
metal oxides they have much lower melting and boiling points. When dissolved in
water the nonmetal oxides form acidic solutions with a pH below 7. There are
multiple oxides of phosphorus, sodium and chlorine but they all react to produce an
acidic species with water.
The equation for phosphorus (V) oxide and water is:
P4O10 + 6H2O \,→\, 4H3PO4
o
Sulfur trioxide reacts to produce sulfuric acid, a reaction which is run on an
industrial scale in the contact process:
SO3 + H2O \,→\, H2SO4
o
In the same manner, chlorine (VII) oxide reacts with water to produce perchloric
acid which is a very strong acid.
Cl2O7 + H2O \,→\, 2HClO4
Chlorine also has the chlorine (I) oxide form, Cl2O which reacts with water to form a
weaker acid, HOCl.
o
This trend of acidic nonmetal oxides is not only in period 3. Oxides of nitrogen like
nitrogen dioxide, NO2, react with water to produce nitric acid, HNO3 and the weak
acid nitrous acid, HNO2:
2 NO2 + H2O \,→\, HNO3 + HNO2
The nitrous acid breaks down over time into more nitric acid and NO gas. Like those
in period 3, nitric acid is a strong acid and will produce very acidic solutions.
From all of this, you can determine that going from left to right across a period,
oxide compounds go from being basic to acidic in nature.