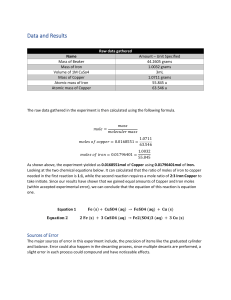
Department of Process Engineering Chemical Engineering D 316 Report 1B: Solvent Extraction Ms. EJ Pool 23631406 Group 12 10 March 2023 © Stellenbosch University Plagiarism Declaration I, A.N. Other (student number), declare that: I have read and understand the Stellenbosch University Policy on Plagiarism and the definitions of plagiarism and self-plagiarism contained in the Policy [Plagiarism: The use of the ideas or material of others without acknowledgement, or the re-use of one’s own previously evaluated or published material without acknowledgement or indication thereof (selfplagiarism or text recycling)]. I also understand that direct translations are plagiarism, unless accompanied by an appropriate acknowledgement of the source. I also know that verbatim copy that has not been explicitly indicated as such, is plagiarism. I know that plagiarism is a punishable offence and may be referred to the University's Central Disciplinary Committee (CDC) who has the authority to expel me for such an offence. I know that plagiarism is harmful for the academic environment and that it has a negative impact on any profession. Accordingly all quotations and contributions from any source whatsoever (including the internet) have been cited fully (acknowledged); further, all verbatim copies have been expressly indicated as such (e.g. through quotation marks) and the sources are cited fully. Except where a source has been cited, the work contained in this assignment is my own work and that I have not previously (in its entirety or in part) submitted it for grading in this module/assignment or another module/assignment. Signed Date i Abstract This practical involves the extraction of copper (II) and iron (III) from a pregnant leach solution which was prepared from Fe3+ solution and CuSO4.5H2O crystals. The provided organic phase is mixed with the aqueous phase (PLS), and an oxime extractant diluted in kerosene is used to facilitate the process. Different organic and aqueous solution ratios are used, and some are done in triplicate. Diluted solutions of the PLS and aqueous solutions were sent to a laboratory to undergo an ICP analysis. The results of these analyses were then used. Three different concentrations of the oxime extractant diluted in kerosene are used (8%, 12%, and 16%) and by analysis of equilibrium curves, regression models, percentage of copper (II) and iron (III) extracted and selectivity towards copper (II), it is found that the extractant concentration best suited to this procedure is the 12% extractant concentration. Furthermore, after analysing the triplicate experimental data points, it is found that the 8 % and 16 % extractant concentration data have a great uncertainty. ii Table of Contents Plagiarism Declaration ............................................................................................................................. i Abstract ................................................................................................................................................... ii Table of Contents ................................................................................................................................... iii Nomenclature ........................................................................................................................................ iv 1 Introduction .................................................................................................................................... 5 2 Theory / Literature .......................................................................................................................... 5 3 4 2.1 Pregnant Leach Solution ......................................................................................................... 5 2.2 Solvent Extraction ................................................................................................................... 5 2.3 ICP spectroscopy analysis for aqueous samples ..................................................................... 6 2.4 Mole balance for determining organic phase concentrations................................................ 6 Methodology................................................................................................................................... 6 3.1 Experimental plan ................................................................................................................... 6 3.2 Equipment and materials ........................................................................................................ 7 Results and Discussion .................................................................................................................... 8 4.1.1 Equilibrium curves for copper and iron at 8 vol%, 12 vol% and 16 vol% oxime diluted in kerosene (extractant) and regression ............................................................................................. 8 4.1.2 Percentage Cu2+ and Fe3+ extracted ................................................................................ 9 4.1.3 Selectivity towards copper (II) ...................................................................................... 10 4.1.4 Performance of the solvent .......................................................................................... 11 5 Conclusions and Recommendations ............................................................................................. 12 6 References .................................................................................................................................... 12 Appendix A. Experimental Results..................................................................................................... 13 Raw data for Copper(ll) and Iron(lll) obtained from ICP analysis ..................................................... 13 Appendix B. Processed Data.............................................................................................................. 15 Equilibrium curve data ...................................................................................................................... 15 Regression data................................................................................................................................. 17 Appendix C. Typical Calculations ....................................................................................................... 18 iii Nomenclature Symbols n mole mol m Mass g V Volume ml Density kg/m3 Greek symbols ρ Subscripts and superscripts aq In the aqueous phase/solution/layer org In the organic phase/solution/layer Acronyms PLS Pregnant Leach Solution ICP Inductively Coupled Plasma iv 1 Introduction When two immiscible solvents differ in their solubility or distribution coefficient, a molecule is extracted from one solvent into another during solvent extraction (H & L, 2017). Applications of this process in industry include water effluent treatment, extraction of rare earth metals, purification of substances, and reprocessing of nuclear waste (D & F, 2022). The two main aims of this practical are understanding a typical solvent extraction procedure and evaluating the extractant's performance in this process. These aims will be met by creating equilibrium curves for copper and iron for the data acquired at different concentrations of the extractant, creating a line of regression, calculating the distribution ratio and percentage extracted of both copper and iron as a function of the O/A ratio tested and discussing the performance of the solvent and its concentration on phase equilibrium as well as the extraction of and selectivity towards copper. 2 Theory / Literature 2.1 Pregnant Leach Solution Pregnant leach solution or PLS is used in solvent extraction and electrowinning processes. For direct manufacturing of high-purity cathodes, the pregnant leach solutions from leaching are excessively diluted and impure. These solutions would produce impure copper deposits after electrowinning. To create pure, high-copper electrolytes from diluted, impure pregnant leach solutions, solvent extraction is used. (WG, 2001) 2.2 Solvent Extraction The PLS is combined with an organic liquid that contains an extractant during solvent extraction. The goal is to promote the development of soluble in the organic phase organometallic complexes between the target metal species in the aqueous phase and the extractant, ensuring that the resulting complex remains in the organic phase. Due to the difference in solubility or distribution coefficient between two immiscible (or barely soluble) solvents, the process of solvent extraction involves moving a chemical from one solvent to another. (M.H.I, 2016) Gravity helps to separate the two phases since the aqueous layer is denser than the organic layer. The preferred extractants for copper extraction are oximes, and equation [1] can be used to define the general reaction wherein the extractant and copper produce the organometallic complex through ion exchange. 2+ +1 [1] Equation 1: 𝐶𝑢𝑎𝑞 + 2𝑅𝐻𝑜𝑟𝑔 ↔ Cu𝑅2,𝑜𝑟𝑔 + 2𝐻𝑎𝑞 5 Where RH denotes the extractant with proton H+, and the subscripts “aq” and “org” denote species in the aqueous and organic phases respectively. 2.3 ICP spectroscopy analysis for aqueous samples An analytical technique called ICP (Inductively Coupled Plasma) Spectroscopy is used to find and quantify components in chemical sample analysis. In this process, a sample is ionized by a very hot plasma, often formed of argon gas. The middle of the plasma is cut through by a gas flow, which results in a channel that is colder than the surrounding plasma but still significantly warmer than a chemical flame. A nebulizer is typically used to generate a liquid mist before releasing the sample into the central channel to be analysed. (F, 2012) 2.4 Mole balance for determining organic phase concentrations In order to create the equilibrium curves, the moles of copper and iron in the organic phase must first be calculated by equation [2] and then converted to concentration. [2] Equation 2: 𝒏𝒐𝒓𝒈 = 𝒏𝑷𝑳𝑺 − 𝒏𝒂𝒒 Equation [3] will be used for extraction isotherms and to assess regression. [3] Equation 3: [𝑪𝒖𝟐+ 𝒂𝒒 ] Where Keq = = [𝑪𝒖𝟐+ (𝒐𝒓𝒈) ] 𝑲𝒆𝒒 ([𝑹𝒕𝒐𝒕𝒂𝒍] − 𝟐[𝑪𝒖𝟐+ (𝒐𝒓𝒈) ]) [𝐶𝑢𝑅2 (𝑜𝑟𝑔)] 2+ [𝐶𝑢 (𝑎𝑞)][𝑅𝐻(𝑜𝑟𝑔)]2 is the equilibrium constant and [𝑅𝑡𝑜𝑡𝑎𝑙 ] = [𝑅𝐻(𝑜𝑟𝑔)] + 2[𝐶𝑢𝑅2 (𝑜𝑟𝑔)] 3 Methodology 3.1 Experimental plan Firstly, the PLS was made as the aqueous layer. This was then mixed with the organic solution. The PLS and organic solution were mixed at different ratios and an oxime extractant diluted in kerosene was added to facilitate with the extractant process. Some of these ratios were done in triplicate to test their repeatability. Samples of the diluted aqueous phase and PLS were then sent to a laboratory for 6 an ICP analysis to determine the concentration of copper and iron in said samples. The results obtained from the ICP analysis is in Appendix A. 3.2 Equipment and materials The equipment required for this practical is as follows: • 5 x magnetic stirrers • 9 x separation funnels • 1 x 50 ml, 1 x 100 ml measuring cylinder (to be used for the aqueous phase only) • 1 x 10 ml, 1 x 50 ml and 1 x 100 mL measuring cylinder (for the organic phase) • 3 x glass funnels • 10 x 100 ml, 10 x 250 ml and 2 x 600 mL glass beakers • 5 x 10 cm watch glasses to cover beakers • 13 x 100 ml volumetric flasks for dilution of samples • 1 x 1000 – 5000 μL variable pipette (and associated tips) • 1 x pH meter • 1x laboratory mass balance The chemicals required for this practical are as follows: • The organic phase: 8 vol%, 12 vol%, and 16 vol% LIX 984N-C (an oxime extractant supplied by BASF) diluted in kerosene. • (Assume that the molecular weight of the organic extractant is 263 and has a density (25 °C) of approximately 0.94 g/cm3 ). • For the aqueous phase prepare approximately 7.4 g/L Cu2+ and 4.5 g/L Fe3+, by addition of CuSO4·5H2O and Fe2(SO4)3·xH2O crystals to demineralized water. • Dilute sulphuric acid (0.05 M H2SO4). • Concentrated sulphuric acid (98% H2SO4). 7 4 Results and Discussion 4.1.1 Equilibrium curves for copper and iron at 8 vol%, 12 vol% and 16 vol% oxime diluted in kerosene (extractant) and regression The aqueous phase concentration of copper and iron was obtained from the ICP analysis. The concentration in the organic phase was calculated using the mole balance, [2] Equation 2. The sample Concentration in organic layer (mol/L) calculations are available in Appendix C. 8 % LIX 12 % LIX 16 % LIX Линейная (8 % LIX) Линейная (12 % LIX) Линейная (16 % LIX) 3,5 3 2,5 2 1,5 1 0,5 0 0 0,001 0,002 0,003 0,004 0,005 0,006 Concentration in aqueous layer (mol/L) Figure 1: Equilibrium curves for copper at 8 vol%, 12 vol% and 16 vol% extractant diluted in kerosene and regression As depicted in Figure 1, the experimental data used for the extractant concentrations of 8% and 16% appear to be spread out and have no apparent linear form. Because of this, the equilibrium experimental data points do not fit the linear regression analysis for the respective extractant concentrations well. However, the experimental data used for the extractant concentration of 12% are less spread out and thus fit the linear regression analysis relatively well. This means that there is a relatively good fit of the linear regression analysis to the experimental data for an extractant concentration of 12%. 8 Concentration in organic layer (mol/L) 8 % LIX 12 % LIX 16 % LIX Линейная (8 % LIX) Линейная (12 % LIX) Линейная (16 % LIX) 2 1,5 1 0,5 0 0 0,001 0,002 0,003 0,004 0,005 0,006 Concentration in aqueous layer (mol/L) Figure 2: Equilibrium curves for Iron at 8 vol%, 12 vol% and 16 vol% extractant diluted in kerosene and regression As depicted in Figure 2, the experimental data used for extractant concentrations of 8% and 16% also appear to be spread out and have no apparent linear form, whilst the experimental data used for an extractant concentration of 16% appear to be less spread out and has some linear form. This means that the equilibrium experimental data used for extractant concentrations of 8% and 16% do not fit the linear regression analysis well, while the data points used for an extractant concentration of 12% has a relatively good fit of the linear regression analysis. 4.1.2 Percentage Cu2+ and Fe3+ extracted Table 1: Percentage of Copper (II) and Iron (III) extracted with respect to the O/A ratios tested using averaged triplicate experimental points. Extractant Concentrations Ratios (O/A) 04:01 03:01 02:01 01:01 01:02 01:03 01:04 8% Cu Fe 98.275293 93.035318 98.178954 92.958318 97.56442 95.342212 97.3879 97.627736 97.038484 97.970526 95.69431 97.440822 97.047119 98.368052 9 12% Cu Fe 98.9206228 94.904544 99.2620457 94.779803 99.1386594 96.481474 98.2157944 97.274975 97.844476 98.108844 97.5451724 98.178626 97.9910803 98.612145 16% Cu Fe 98.257193 97.7729951 97.935085 96.4800663 97.819522 97.0606108 96.864251 96.9539563 98.119231 98.7144169 95.915262 97.2707471 98.26008 98.8542166 Table 2: Percentage of Copper (II) and Iron (III) extracted with respect to the O/A ratios tested for the triplicate experimental data points. Triplicate ratios (O/A) Extractant Concentrations 1 2 3 Difference between largest and smallest value 01:02 01:02 01:04 8% 12% 16% Cu Fe Cu Fe Cu Fe 96.831317 97.803069 97.9545918 98.21286 98.136314 98.801414 97.571241 98.327083 97.7920014 98.052477 98.135934 98.742738 96.712894 97.781427 97.786835 98.061193 98.507993 99.0184978 0.8583466 0.5456555 0.16775679 0.1603831 0.3720584 Table 1 shows that when the ratio of organic to aqueous phase tested is greater than one, the percentage of copper (II) extracted is greater than that of iron (III). When the ratio is greater than or equal to one, the extractant percentage of copper (II) is only greater than that of iron (III) for an extractant concentration of 12 %. According to Table 2, the triplicate experimental data points at an extractant concentration of 8% vary greatly. At 16 %, they vary less and at 12 % the triplicate experimental data points are the most consistent and similar. At an extractant concentration of 12 % the difference between the largest and smallest data point is the smallest at roughly 0.17 and 0.16 for copper (II) and iron (III) respectively. 4.1.3 Selectivity towards copper (II) Ratios Table 3: Distribution ratios using averaged triplicate experimental data points. Extractant Concentrations 8% 12% 16% 04:01 03:01 02:01 01:01 01:02 01:03 01:04 5.064011469 4.84913592 2.396379687 1.136030909 0.85703104 0.742288543 0.690109062 5.915937556 8.866610998 5.119203665 1.912422745 1.097321568 0.927639153 0.86354392 1.598624495 2.133693727 1.687286787 1.215409991 0.854506615 0.834874926 0.823042885 10 0.27575987 Table 4: Distribution ratios for the triplicate experimental data points. Triplicate ratios (O/A) Extractant Concentrations 1 2 3 Difference between largest and smallest value 01:02 01:02 01:04 8% 12% 16% 0.866584 1.0928759 0.80377742 0.8610087 1.1033059 0.84305269 0.8435005 1.0957829 0.82229854 0.0230835 0.01043 0.03927527 Table 3 indicates that the 16 % extractant concentration has the worst selectivity due to its low distribution ratios, an 8 % extraction concentration has a better selectivity, and a 12 % extractant concentration has the best selectivity given its high distribution ratios. With reference to Table 4, the 12 % extractant concentration has the smallest difference of approximately 0.01 between the largest and smallest triplicate experimental data points. 4.1.4 Performance of the solvent Based on Table 2 and Table 4’s findings, the 8 % and 16 % extractant concentrations have the greatest uncertainty and thus a 12 % extractant concentration would have the most accurate data. Given that, for an extractant concentration of 12 %, the percentage of copper (II) extracted is the highest (Table 1), the selectivity is the highest (Table 3) and the uncertainty based on the triplicate experimental data points is the lowest (Table 2 and Table 4), it can be said that an extractant concentration of 12 % would be the best choice. Furthermore, the equilibrium curves for copper (II) and iron (III) depicted in Figure 1 and Figure 2 respectively, show that the equilibrium experimental data used for an extractant concentration of 12 % has a relatively good fit of the linear regression analysis. This supports the extraction concentration of 12 % as the best choice. 11 5 Conclusions and Recommendations After evaluating the equilibrium curves, regression models, percentage of copper and iron extracted as well as the selectivity towards copper it was concluded that the extractant concentration best suited to this procedure was the 12% concentration. The other equilibrium curves and regression models of the remaining extractant concentrations had a poor fit and were not suited to this procedure. Furthermore, it was found that the 8 % and 16 % extractant concentrations had the greatest uncertainty after evaluating the triplicate experimental data points. The percentage of copper (II) extracted as well as the selectivity using the 12 % extractant concentration was the highest. Recommendations for this practical would be that a longer period than 10 minutes be set out for equilibrium to be reached so that better separation and extraction can occur. 6 References Smith, D. & Adams, F. (2022, July 2). Solvent Extraction: Procedures and Applications. Retrieved from PSIBERG: https://psiberg.com/solvent-extraction/ Grimwade, F . (2012, March 11). What is ICP Spectroscopy? Retrieved from XRF Scientific: https://www.xrfscientific.com/what-is-icp-spectroscopy/ Chen, H. & Wang, L. (2017, March 2). Solvent Extraction. Retrieved from Science Direct: https://www.sciencedirect.com/topics/engineering/solventextraction#:~:text=Solvent%20extraction%20is%20the%20process,(or%20slightly%20soluble )%20solvents. Baird, M, H, I. (2016, May 16). Solvent Extraction. Retrieved from Science Direct: https://www.sciencedirect.com/topics/engineering/solventextraction#:~:text=Solvent%20extraction%20is%20the%20process,(or%20slightly%20soluble )%20solvents. Davenport, W, G. (2001, February 2). Pregnant Leach Solution. Retrieved from Science Direct: https://www.sciencedirect.com/topics/engineering/pregnant-leach-solution 12 Appendix A. Experimental Results Raw data for Copper(ll) and Iron(lll) obtained from ICP analysis Table 5: Concentrations of copper(ll) and iron(lll) in the aqueous layer for different O/A ratios and their averages. G7 G12 G11 Ratios 01:02 01:02 01:02 01:01 01:03 01:04 02:01 03:01 04:01 PLS 1 PLS 2 PLS 3 04:01 03:01 02:01 01:01 01:02 01:03 01:04 01:04 01:04 PLS 1 PLS 2 PLS 3 04:01 03:01 02:01 01:01 01:02 01:02 01:02 01:03 01:04 PLS 1 PLS 2 Cu 324,752 Cu 327,393 (mg/L) (mg/L) 140.877 140.638 107.765 108.013 146.067 145.969 77.417 77.294 191.85 190.68 175.39 174.4 54.102 54.09 27.054 26.875 25.617 25.459 205.29 205.06 126.589 127.157 146.593 146.63 25.911 25.701 30.695 30.456 48.486 48.374 92.84 92.886 83.624 83.469 182.01 180.89 110.26 110.507 110.2 110.612 88.424 88.315 55.942 55.837 65.257 65.013 68.042 67.824 16.035 15.93 10.979 10.875 19.156 19.106 52.858 52.818 90.813 90.907 98.045 98.12 98.242 98.382 108.807 109.287 118.691 119.28 71.02 70.63 147.654 147.512 Average Cu 140.7575 107.889 146.018 77.3555 191.265 174.895 54.096 26.9645 25.538 205.175 126.873 146.6115 25.806 30.5755 48.43 92.863 83.5465 181.45 110.3835 110.406 88.3695 55.8895 65.135 67.933 15.9825 10.927 19.131 52.838 90.86 98.0825 98.312 109.047 118.9855 70.825 147.583 13 Fe 259,939 Fe 239,562 Fe 238,204 (mg/L) (mg/L) (mg/L) 59.441 59.359 59.151 45.28 45.177 45.049 60.008 59.925 59.771 42.716 42.726 42.66 69.168 69.114 69.011 58.834 58.773 58.643 62.834 62.768 63.038 63.206 63.293 63.626 62.506 62.678 62.862 62.234 62.279 62.082 38.165 38.15 38.006 43.836 43.826 43.653 20.059 20.058 20.012 31.675 31.689 31.674 39.601 39.666 39.778 54.672 55.001 54.813 34.683 34.813 34.636 73.357 73.917 73.795 43.027 43.208 43.212 45.136 45.312 45.336 35.291 35.397 35.314 17.187 17.15 17.131 20.385 20.37 20.338 21.417 21.492 21.367 45.831 45.667 46.079 46.948 46.946 47.051 47.68 46.904 47.916 48.808 49.055 49.288 48.134 48.393 48.231 52.482 52.69 52.577 52.24 52.507 52.296 49.103 49.236 49.192 49.812 50.03 50.046 22.186 22.161 22.151 46.021 46.026 45.839 Average Fe 59.317 45.168667 59.901333 42.700667 69.097667 58.75 62.88 63.375 62.682 62.198333 38.107 43.771667 20.043 31.679333 39.681667 54.828667 34.710667 73.689667 43.149 45.261333 35.334 17.156 20.364333 21.425333 45.859 46.981667 47.5 49.050333 48.252667 52.583 52.347667 49.177 49.962667 22.166 45.962 PLS 3 167.28 166.31 166.795 14 50.573 50.688 50.36 50.540333 Appendix B. Processed Data Equilibrium curve data Table 6: Concentration of organic phase for copper and iron at an extractant percentage of 8% Organic phase concentration Cu Fe 1.935104 1.070859426 1.936828 1.071703927 1.934828 1.070824548 1.453822 0.803888429 2.898682 1.605413442 2.89997 1.606339905 0.969825 0.535323377 0.970536 0.535308604 0.72793 0.401496965 Table 7: Concentration of organic phase for copper and iron at an extractant percentage of 12% Cu 0.727925 0.970441 0.969973 1.453212 1.938104 2.899454 1.936697 2.905043 2.906777 Fe 0.402451 0.536255 0.536016 0.803345 1.072328 1.605002 1.607737 1.607548 1.608436 Table 8: Concentration of organic phase for copper and iron respectively at an extractant percentage of 16% Cu 0.728118 0.970957 0.970742 1.454786 1.937721 2.906013 1.93733 2.90515 Fe 0.401874 0.535798 0.535782 0.803604 1.07152 1.071261 1.071275 1.607197 15 2.904368 1.607127 Table 9: Concentration of aqueous phase for copper and iron respectively at an extractant percentage of 8% Cu 0.003692 0.00283 0.003829 0.003043 0.005016 0.00344 0.002837 0.000707 0.002009 Fe 0.00177 0.001348 0.001788 0.001912 0.002062 0.001315 0.003753 0.001891 0.005612 Table 10: Concentration of aqueous phase for copper and iron respectively at an extractant percentage of 12% Cu 0.001257 0.00086 0.001003 0.002079 0.002383 0.002572 0.002578 0.00286 0.00234 Fe 0.004106 0.004206 0.002835 0.002196 0.00144 0.001569 0.001562 0.001468 0.001118 Table 11: Concentration of aqueous phase for copper and iron respectively at an extractant percentage of 16% Cu 0.00203 0.002406 0.00254 0.003653 0.002191 0.004759 0.002171 0.002172 0.001738 Fe 0.001795 0.002836 0.002369 0.002455 0.001036 0.002199 0.000966 0.001013 0.000791 16 Regression data Table 12: Keq values for copper and iron respectively at an extraction percentage of 8 % Cu Fe 2.03E+02 1.12E+02 2.03E+02 1.12E+02 2.03E+02 1.12E+02 1.53E+02 8.44E+01 3.04E+02 1.68E+02 3.04E+02 1.69E+02 1.02E+02 5.62E+01 1.02E+02 5.62E+01 7.64E+01 4.21E+01 Table 13: Keq values for copper and iron respectively at an extraction percentage of 12 % Cu Fe 3.40E+01 1.87E+01 4.53E+01 2.50E+01 4.53E+01 2.50E+01 6.79E+01 3.75E+01 9.04E+01 5.00E+01 1.36E+02 5.00E+01 9.04E+01 5.00E+01 1.36E+02 7.50E+01 1.35E+02 7.50E+01 Table 14: Keq values for copper and iron respectively at an extraction percentage of 16 % Cu Fe 1.91E+01 1.06E+01 2.55E+01 1.41E+01 2.55E+01 1.41E+01 3.81E+01 2.11E+01 5.09E+01 2.81E+01 7.61E+01 4.21E+01 5.08E+01 4.22E+01 7.62E+01 4.22E+01 7.63E+01 4.22E+01 17 Appendix C. Typical Calculations Aqueous solution (PLS): m(Cu2+) = 14.55g × 63.55 249.68 = 3.703 g 𝑛(𝐶𝑢2 +) = 0.0582746 𝑚𝑜𝑙 𝑛(𝐹𝑒3 +) = 0.08058 𝑚𝑜𝑙 × 0.4𝑑𝑚3 = 0.032232𝑚𝑜𝑙 𝑑𝑚3 Aqueous phase: [𝐶𝑢2 +] = 140.7575 𝑚𝑔 1 × 10−3 𝑔 × = 0.1407575𝑔/𝐿 𝐿 𝑚𝑔 𝑚(𝐶𝑢2+) = 0.1407575 𝑔/𝐿 × 0.1 𝐿 = 0.01407575 𝑔 𝑛(𝐶𝑢2+) = 𝑚(𝐶𝑢2+) = 2.2149 × 10−4 𝑚𝑜𝑙 63.55 Same procedure for Fe3+ Mole Balance: 𝑛𝑜𝑟𝑔 = 𝑛𝑃𝐿𝑆 − 𝑛𝑎𝑞 𝑛𝑜𝑟𝑔 (𝐶𝑢2+) = 0.0582746 𝑚𝑜𝑙 − 2.2149 × 10−4 𝑚𝑜𝑙 = 0.05805311 𝑚𝑜𝑙 Convert back to concentration using ratios of volumes given: 𝐶 = [Cu2+] = 0.05805311 mol/ 0.03 L = 1.935 M 18 𝑛 𝑉 19 20