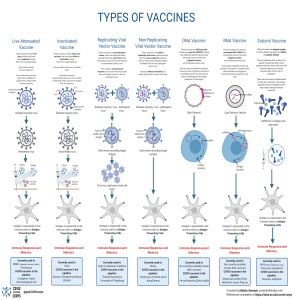
Title: Drug Discovery and Development for COVID-19 Vaccine Abstract: The emergence of COVID-19, caused by the novel coronavirus SARS-CoV-2, posed a significant global health challenge. Drug discovery and development efforts played a crucial role in the fight against this pandemic. This assignment explores the process of drug discovery and development for COVID-19, focusing on key targets, methodologies, and approved therapies. It also highlights the challenges faced during this process and potential future directions. The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has posed an unprecedented global health crisis. The urgent need for effective treatments and vaccines led to a rapid and collaborative effort in drug discovery and development. This paper presents a comprehensive overview of the various stages involved in the process of identifying and developing drugs for COVID-19. Starting with the initial understanding of the virus and its pathogenesis. Additionally, we explore the challenges faced, success stories, and future prospects for combating COVID-19. 1. Introduction: Overview of COVID-19 and its impact on global health and other sector: COVID-19, short for "Coronavirus Disease 2019," is a highly contagious respiratory illness caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The virus was first identified in December 2019 in the city of Wuhan, Hubei province, China, and quickly spread to become a global pandemic. It is primarily transmitted through respiratory droplets when an infected person coughs, sneezes, or talks, and can also spread by touching surfaces contaminated with the virus and then touching the face, particularly the mouth, nose, or eyes. Impact on Global Health: The emergence of COVID-19 as a global health crisis has had significant implications on public health, economies, and societies worldwide. Below are some of the key impacts: I. Health Systems Overwhelmed: The rapid spread of COVID-19 overwhelmed healthcare systems in many countries, leading to shortages of medical equipment, hospital beds, and healthcare personnel. This, in turn, resulted in challenges in providing adequate care to all patients, not only those with COVID-19 but also those with other medical conditions. ii. High Mortality Rates: COVID-19 has caused a considerable number of deaths globally. Vulnerable populations, such as the elderly and those with underlying health conditions, are at a higher risk of severe illness and mortality. iii. Social and Economic Disruptions: Governments around the world implemented various measures to contain the spread of the virus, including lockdowns, travel restrictions, and the closure of businesses and schools. These measures had severe economic consequences, with millions of people losing their jobs and businesses facing financial hardships. iv. Mental Health Impact: The pandemic's isolation, fear, and uncertainty have taken a toll on people's mental health. Anxiety, depression, and stress levels have increased, and mental health services have seen a surge in demand. v. Educational Disruptions: School closures and the shift to remote learning have disrupted education globally. Many students faced challenges accessing online education due to technology limitations or lack of a conducive learning environment at home. vi. Research and Vaccine Development: The pandemic has spurred an unprecedented effort in scientific research and vaccine development. Collaborative efforts led to the rapid development and approval of multiple COVID-19 vaccines, setting a new record for vaccine development. vii. Global Cooperation and Information Sharing: COVID-19 highlighted the importance of global cooperation in responding to public health emergencies. Countries and organizations worldwide shared information, data, and research findings to enhance understanding of the virus and develop effective response strategies. viii. Inequality Exacerbated: The pandemic disproportionately affected vulnerable and marginalized populations, including low-income communities, minorities, and refugees. Existing inequalities in access to healthcare and resources were exacerbated during the crisis. ix. Disruptions to Essential Services: Non-COVID-19 medical services, such as routine vaccinations and elective surgeries, were disrupted during the pandemic, leading to potential long-term health consequences. x. Long-term Health Effects: Some COVID-19 survivors experienced long-term health effects known as "long COVID," with symptoms persisting for months after their initial infection. The urgency of drug discovery efforts to combat the pandemic The urgency of drug discovery efforts to combat the COVID-19 pandemic cannot be overstated. The rapid and global spread of the SARS-CoV-2 virus, combined with its high infection rates and severe health implications, has made it imperative to develop effective treatments as quickly as possible. Several factors contribute to the urgency of drug discovery in this context: i. Public Health Crisis: COVID-19 has evolved into an unprecedented public health crisis, overwhelming healthcare systems worldwide. The number of infections and deaths has risen rapidly, putting immense strain on medical facilities and healthcare workers. Developing effective drugs to treat and manage COVID-19 is essential to reduce the burden on healthcare systems and save lives. ii. Global Impact: The pandemic's global impact has been far-reaching, affecting virtually every country, community, and individual. The social and economic consequences have been severe, with lockdowns and travel restrictions disrupting daily life and economies. Finding effective treatments is critical to controlling the spread of the virus and facilitating a return to normalcy. iii. High Mortality Rates: COVID-19 has resulted in a significant number of deaths globally, especially among vulnerable populations. While vaccines play a crucial role in prevention, drug development is essential to treat those infected, reduce mortality rates, and alleviate the severity of the disease. iv. Emerging Variants: SARS-CoV-2 continues to mutate and produce new variants, some of which have demonstrated increased transmissibility or resistance to existing treatments and vaccines. The ongoing evolution of the virus necessitates the development of adaptable drugs to effectively combat these variants. v. Overcoming Challenges: Drug development is a complex and time-consuming process that typically takes years. However, due to the urgency of the pandemic, researchers have accelerated the process by leveraging advanced technologies and international collaboration. The rapid development and approval of effective drugs can significantly impact the course of the pandemic. vi. Diversifying Treatment Options: While vaccines are essential for prevention, not everyone can receive or respond to vaccination adequately. Developing a range of effective drugs offers diverse treatment options for different patient populations, including those with compromised immune systems or allergies to vaccines. vii. Addressing Long COVID: Some individuals experience prolonged symptoms and health effects after recovering from COVID-19, known as "long COVID." Drug discovery efforts must also focus on finding treatments for these persistent health issues to improve the quality of life for affected individuals. viii. Preparedness for Future Pandemics: The COVID-19 pandemic serves as a wake-up call for the importance of preparedness for future infectious disease outbreaks. Investments in drug discovery research and infrastructure will enhance our ability to respond swiftly and effectively to any future pandemics. ix. Resilience in the Face of Variability: Even with widespread vaccination, breakthrough infections and cases in unvaccinated populations will continue to occur. Having effective drugs to treat COVID-19 will be crucial in mitigating outbreaks and maintaining population health. x. Global Collaboration: The urgency of the pandemic has led to unprecedented global collaboration among scientists, researchers, pharmaceutical companies, and regulatory bodies. This spirit of cooperation can pave the way for more effective and efficient drug development processes in the future. 2. Targets for Drug Development Drug development for COVID-19 and other infectious diseases involves targeting specific components of the virus or the host's immune response to either inhibit viral replication or modulate the immune response. Some of the key targets for drug development against COVID-19 include: i. **Viral Proteins:** a. **Main Protease (Mpro):** This enzyme is crucial for viral replication. Inhibiting Mpro can block viral replication and the production of new viral particles. b. **RNA-dependent RNA Polymerase (RdRp):** RdRp is responsible for replicating the viral RNA genome. Inhibiting RdRp can hinder viral replication. c. **Spike Protein:** The spike protein enables the virus to enter host cells. Targeting the spike protein can prevent viral entry and infection. ii. **Host Cell Receptors and Proteins:** a. **ACE2 Receptor:** SARS-CoV-2 enters host cells through binding to the ACE2 receptor. Drugs that block this interaction may prevent viral entry. b. **TMPRSS2:** TMPRSS2 is a host protease that primes the spike protein for cell entry. Inhibiting TMPRSS2 can potentially prevent viral fusion with host cells. iii. **Inflammatory Response Modulators:** a. **Cytokine Inhibitors:** Some severe COVID-19 cases exhibit a cytokine storm, an overactive immune response. Targeting specific cytokines can help manage inflammation. b. **Janus Kinase (JAK) Inhibitors:** These drugs can suppress inflammatory responses by targeting JAK signaling pathways. iv. **Monoclonal Antibodies:** a. **Neutralizing Antibodies:** Monoclonal antibodies that directly target the virus can neutralize it, reducing viral load and disease severity. b. **Anti-Inflammatory Antibodies:** Some monoclonal antibodies can modulate the immune response, potentially reducing excessive inflammation. v. **RNA Interference (RNAi):** RNAi-based therapies can interfere with viral RNA, preventing viral replication. vi. **Antiviral Drugs:** a. **Remdesivir:** An antiviral drug that inhibits viral replication by interfering with RdRp activity. b. **Favipiravir:** Another antiviral drug that inhibits RdRp and disrupts viral replication. vii. **Immunomodulatory Drugs:** Drugs that modulate the immune response to prevent hyperinflammation or boost the body's immune defenses. viii. **Protease Inhibitors:** Drugs that inhibit viral proteases, preventing viral replication. ix. **Vaccines:** Although not drugs, vaccines are essential tools for preventing infection by training the immune system to recognize and respond to the virus. It's important to note that drug development is a complex process, and not all potential drug candidates targeting these components may ultimately be successful. Additionally, combination therapies may be necessary to address the multifaceted nature of COVID-19 and to combat potential drug resistance. 3. Drug Discovery Methods COVID-19 drug discovery methods involve a multi-faceted approach that combines various strategies to identify potential therapeutic agents to combat the virus. Here are some of the key methods used in COVID-19 drug discovery: i. **Repurposing Existing Drugs:** One of the fastest ways to find potential treatments for COVID-19 was to investigate existing drugs that were already approved for other diseases. Researchers screened libraries of approved drugs to identify candidates that could be repurposed for treating COVID-19 based on their known mechanisms of action. ii. **In Silico Drug Discovery:** This method involves using computational tools and algorithms to simulate interactions between potential drugs and the SARS-CoV-2 virus. Virtual screening techniques, molecular docking, and molecular dynamics simulations are used to identify molecules that could bind to viral proteins and inhibit their function. iii. **High-Throughput Screening (HTS):** HTS is a laboratory method used to quickly test large numbers of chemical compounds for their activity against the virus. It allows researchers to identify potential drug candidates more rapidly by testing thousands of compounds simultaneously. iv. **Antiviral Screening:** Researchers isolated the virus and established viral cultures in laboratories, enabling them to test potential antiviral compounds directly against the virus to determine their efficacy in stopping viral replication. v. **Monoclonal Antibodies:** Monoclonal antibodies are laboratory-produced molecules designed to mimic the body's immune system's ability to fight off harmful pathogens. Researchers identified and tested monoclonal antibodies that target the SARS-CoV-2 spike protein, which the virus uses to enter human cells. vi. **Convalescent Plasma:** Convalescent plasma therapy involves using blood plasma from individuals who have recovered from COVID-19 and have developed antibodies against the virus. This plasma, rich in antibodies, is transfused into critically ill patients to help them fight the infection. vii. **RNA-Targeted Therapies:** Since SARS-CoV-2 is an RNA virus, researchers explored the development of RNA-targeted therapies, such as RNA interference (RNAi) and antisense oligonucleotides, to disrupt viral replication. viii. **Cell-Based Assays:** These assays involve testing the potential drugs on human cells infected with the virus to study their effects on viral replication and the host cell response. ix. **Animal Studies:** Before testing potential drugs on humans, researchers conducted preclinical studies in animal models infected with the virus to assess their safety and efficacy. x. **Clinical Trials:** Once potential drug candidates were identified through various methods, they underwent rigorous clinical trials to evaluate their safety and effectiveness in human patients. It's important to note that drug discovery is a complex and iterative process. Many potential drug candidates may not progress past certain stages due to safety or efficacy concerns. The development of effective COVID-19 treatments requires a collaborative effort between researchers, pharmaceutical companies, regulatory authorities, and healthcare professionals. As the pandemic evolves, ongoing research and development efforts continue to refine treatment options for COVID-19. 4. Major Antiviral Therapies Antiviral therapies involve the development of drugs that directly target the virus, inhibiting its replication and spread within the body. These drugs are used to treat individuals who have already been infected with the virus. Some of the major antiviral therapies used or explored for COVID-19 include: a. **Remdesivir:** Remdesivir is an antiviral drug that was originally developed to treat Ebola. It has shown some effectiveness in shortening the recovery time for COVID-19 patients, particularly in hospitalized patients with severe disease. b. **Monoclonal Antibodies:** Monoclonal antibodies are laboratory-produced molecules that mimic the body's natural immune response. Several monoclonal antibody therapies have been developed to target the spike protein of SARS-CoV-2, reducing viral load and severity of illness in high-risk patients. c. **Convalescent Plasma:** Convalescent plasma therapy involves using blood plasma from individuals who have recovered from COVID-19 and have developed antibodies against the virus. This plasma is transfused into critically ill patients to provide passive immunity and help fight the infection. d. **Antiviral Drug Combinations:** Some studies explored combinations of antiviral drugs to potentially increase their effectiveness against the virus. 2. **Vaccine Development:** Vaccines are designed to prevent infection by training the immune system to recognize and mount a defense against the virus. Vaccines have been crucial in the global effort to control the spread of COVID-19. Several vaccines have been developed and authorized for emergency use or fully approved by various regulatory agencies worldwide. Some of the major COVID-19 vaccines include: a. **Pfizer-BioNTech (Comirnaty):** An mRNA-based vaccine that trains the immune system to produce antibodies against the spike protein of the virus. b. **Moderna:** Another mRNA-based vaccine that works similarly to the Pfizer-BioNTech vaccine. c. **AstraZeneca (Vaxzevria) and Johnson & Johnson (Janssen):** Viral vector vaccines that use a harmless adenovirus to deliver genetic material that codes for the spike protein. d. **Sinovac (CoronaVac) and Sinopharm:** Inactivated virus vaccines that contain SARS-CoV-2 particles that have been rendered non-infectious. e. **Novavax:** A protein subunit vaccine that contains recombinant spike protein. It's important to note that while vaccines are primarily used for prevention, some studies also explore their potential use as post-exposure prophylaxis or for individuals with prior COVID-19 infection. Both antiviral therapies and vaccines play critical roles in controlling the COVID-19 pandemic, and ongoing research continues to refine and expand our arsenal against the virus. 5. Vaccines vs. Therapeutics Vaccines and therapeutics are two distinct approaches in combating infectious diseases like COVID-19. While they share the common goal of preventing or treating the disease, they work differently and have specific roles in managing the spread and impact of the virus. **Vaccines:** a. **Preventative Approach:** Vaccines are primarily used as a preventive measure to protect individuals from getting infected with the virus in the first place. They train the immune system to recognize and respond to specific pathogens, such as SARS-CoV-2 (the virus that causes COVID-19), without causing the disease itself. b. **Generating Immunity:** When a person receives a vaccine, their immune system recognizes the harmless components of the virus (e.g., viral proteins) introduced by the vaccine and produces antibodies and immune cells that can neutralize the virus if encountered in the future. c. **Population-Level Protection:** Widespread vaccination can lead to herd immunity, where a significant portion of the population is immune to the virus. This reduces the likelihood of widespread transmission, protecting both vaccinated individuals and those who cannot be vaccinated due to medical reasons. d. **Types of Vaccines:** Different COVID-19 vaccines, such as mRNA vaccines, viral vector vaccines, inactivated virus vaccines, and protein subunit vaccines, have been developed and authorized for emergency use or fully approved by various regulatory agencies worldwide. **Therapeutics (Antiviral Therapies):** a. **Treatment Approach:** Therapeutics, also known as antiviral therapies, are drugs or treatments used to treat individuals who have already been infected with the virus. These drugs target the virus directly, inhibiting its replication and spread within the body. b. **Reducing Severity and Duration:** Antiviral therapies aim to reduce the severity of the illness, shorten the duration of the infection, and prevent complications in individuals who have already contracted the virus. c. **Monoclonal Antibodies:** Some therapeutic approaches involve using laboratory-produced monoclonal antibodies, which mimic the body's immune response and can neutralize the virus in infected individuals. d. **Repurposed Drugs:** Some existing drugs originally developed for other purposes (e.g., remdesivir) have been repurposed to treat COVID-19 due to their potential antiviral properties. In summary, vaccines are primarily used for prevention and generate immunity against the virus, while therapeutics are used for treatment in individuals who have already contracted the virus. Both approaches are essential in managing the COVID-19 pandemic and reducing its impact on public health. Vaccines play a crucial role in preventing widespread transmission, while therapeutics help in managing and treating the disease in infected individuals. 6. Challenges in Drug Development for COVID-19 Drug development for COVID-19 faced numerous challenges due to the urgency and complexity of the pandemic. Some of the major challenges include: a. **Rapid Pace:** Developing safe and effective drugs typically takes years or even decades. However, due to the urgency of the pandemic, there was immense pressure to accelerate the process and develop treatments in record time. b. **Clinical Trials:** Conducting rigorous clinical trials is essential to determine the safety and efficacy of drugs. Designing, recruiting participants, and carrying out these trials during a pandemic presented logistical and ethical challenges. c. **Diversity of Patients:** COVID-19 affects individuals differently, with variations in symptoms and severity across different age groups, genders, and comorbidities. It was challenging to ensure that drug trials included a diverse range of participants to understand how the drugs would work in various populations. d. **Evolution of the Virus:** The SARS-CoV-2 virus undergoes mutations, leading to the emergence of different variants. This posed challenges in developing drugs that would remain effective against evolving strains of the virus. e. **Unknown Disease Mechanisms:** COVID-19 was a new disease caused by a novel virus. Understanding the virus's behavior and the disease's pathophysiology was a significant challenge in identifying appropriate drug targets. f. **Drug Repurposing:** Repurposing existing drugs can expedite the drug development process. However, identifying which drugs would be effective against COVID-19 required extensive screening and testing of a large number of compounds. g. **Supply Chain Disruptions:** The pandemic disrupted global supply chains, affecting the availability of raw materials and hindering the manufacturing and distribution of potential drugs. h. **Limited Resources:** Developing drugs and conducting clinical trials require substantial financial and human resources. Competition for funding and research personnel was intense during the pandemic. i. **Regulatory Hurdles:** While the need for speed was critical, maintaining high regulatory standards for drug approval was also crucial to ensure safety and efficacy. j. **Antiviral Resistance:** The potential emergence of antiviral resistance was a concern, necessitating constant monitoring of drug efficacy and adaptation if needed. Despite these challenges, the global scientific community collaborated to make significant strides in drug development for COVID-19. Several treatments, such as remdesivir and monoclonal antibodies, were authorized for emergency use or full approval, providing healthcare professionals with valuable tools to manage and treat COVID-19 patients. Ongoing research continues to refine and improve therapeutic options to combat the virus effectively. 7. Global Collaboration in Drug Development Global collaboration in drug development is a critical aspect of addressing global health challenges like COVID-19. The scale and complexity of the pandemic necessitated a collaborative approach that involved various stakeholders from different countries and organizations. Some of the key aspects of global collaboration in drug development are: a. **Sharing Data and Knowledge:** Scientists and researchers from different countries shared data, research findings, and knowledge about the virus, disease mechanisms, and potential drug targets. Open sharing of information accelerated research efforts and allowed researchers to build upon each other's work. b. **Clinical Trials Collaboration:** International collaboration facilitated the design and execution of large-scale, multi-center clinical trials. These trials involved participants from multiple countries, enabling researchers to collect diverse data and improve the generalizability of the results. c. **Public-Private Partnerships:** Governments, academic institutions, and pharmaceutical companies collaborated in public-private partnerships to pool resources and expertise. Such collaborations accelerated drug development by leveraging the strengths of each partner. d. **Funding and Investment:** Global collaboration allowed for the pooling of financial resources from various governments, organizations, and foundations. This funding supported research, clinical trials, and manufacturing of potential drugs. e. **Regulatory Harmonization:** Regulatory agencies from different countries worked together to harmonize approval processes and expedite the review of potential drugs. Collaborative efforts ensured that necessary safety and efficacy standards were met while avoiding unnecessary delays. f. **Access to Samples and Strains:** Countries shared samples of the virus and its variants to aid in research and development efforts. Access to diverse strains helped in testing the effectiveness of potential drugs against different virus mutations. g. **Capacity Building:** Collaborative efforts included building and strengthening healthcare infrastructure and research capabilities in resource-limited regions. This helped ensure that drug development and healthcare interventions were accessible globally. h. **Manufacturing and Distribution:** Collaboration among pharmaceutical companies and governments facilitated the manufacturing and distribution of drugs to ensure equitable access to treatments worldwide. i. **Global Health Organizations:** International organizations, such as the World Health Organization (WHO), played a crucial role in coordinating and supporting global collaborative efforts in drug development. They provided guidance, shared best practices, and helped align efforts to tackle the pandemic collectively. j. **Public Engagement and Advocacy:** Global collaboration involved engaging with the public, raising awareness, and addressing vaccine hesitancy and misinformation to foster public trust and cooperation in drug development and vaccination efforts. Overall, global collaboration in drug development for COVID-19 exemplified the power of cooperation and collective action in addressing a global health crisis. The lessons learned from this collaborative approach will likely inform future efforts to combat emerging infectious diseases and other health challenges on a global scale. 8. Future Perspectives Future perspectives in drug development and global health, particularly in light of the COVID-19 pandemic, are shaped by the lessons learned and advancements made during this unprecedented time. Here are some key future perspectives: a. **Pandemic Preparedness:** The COVID-19 pandemic exposed the importance of global pandemic preparedness. Governments, international organizations, and healthcare systems will likely invest more in preparedness strategies, early warning systems, and research to respond swiftly and effectively to future pandemics. b. **Vaccine Development Platforms:** The success of mRNA-based vaccines for COVID-19 has highlighted the potential of this technology for developing vaccines against other infectious diseases. This may spur further investment and research in mRNA and other novel vaccine platforms. c. **Drug Repurposing and Drug Discovery:** The experience with drug repurposing during COVID-19 will likely encourage further exploration of existing drugs for new therapeutic indications. Additionally, the accelerated development of new antiviral drugs will continue to be a focus, aiming to target various viral diseases. d. **Global Collaboration and Information Sharing:** The success of global collaboration in addressing the pandemic emphasizes the importance of continued international cooperation in health research, data sharing, and resource mobilization to address future health challenges effectively. e. **Vaccine Equity:** The pandemic highlighted the need for equitable access to vaccines and healthcare globally. Future efforts will likely focus on ensuring fair distribution of vaccines and medical resources, particularly for low- and middle-income countries. f. **Digital Health and Telemedicine:** The pandemic accelerated the adoption of digital health technologies and telemedicine. In the future, these technologies will likely play a more significant role in healthcare delivery, remote monitoring, and patient care. g. **One Health Approach:** The One Health approach, which recognizes the interconnectedness of human, animal, and environmental health, will gain further prominence. This integrated approach is crucial for monitoring and preventing future zoonotic disease outbreaks. h. **Viral Surveillance and Sequencing:** The pandemic underscored the importance of comprehensive viral surveillance and sequencing to track virus variants and understand their implications for disease spread and vaccine efficacy. This will be crucial for managing future outbreaks. i. **Personalized Medicine:** Advancements in genetic sequencing and precision medicine may enable more personalized treatments tailored to an individual's genetic makeup and disease characteristics. j. **Climate Change and Health:** The impact of climate change on health, including its potential to influence the emergence of infectious diseases, will be an area of increasing focus. Strategies to mitigate climate-related health risks will become more critical. k. **Mental Health Support:** The pandemic's toll on mental health has highlighted the need for enhanced mental health support and services, which will likely continue to be a priority in the postpandemic world. 9. Conclusion The impact of drug discovery efforts on managing COVID-19 has been significant, contributing to mitigating the severity of the pandemic and saving countless lives. The rapid development and approval of treatments have provided healthcare professionals with essential tools to combat the virus and improve patient outcomes. Here are some key points regarding the impact of drug discovery efforts and the ongoing challenges: