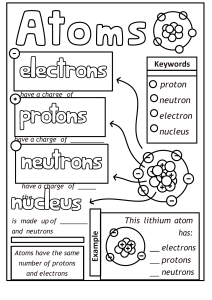
Week 4: Atoms and Elements An element is a pure substance that cannot be broken down into anything simpler. Everything on Earth is made from about one hundred different elements. An atom is the smallest particle of an element. They are much too small to be seen even with the most powerful microscope. Each element contains only one type of atoms. Composition and Structure of the Atom Atoms have a nucleus in its centre with electrons moving around it like planets move around the Sun. The nucleus of an atom houses the protons and neutrons. Except hydrogen atoms which are composed of a proton and a neutron each, atoms of other elements are made up of three sub-atomic particles. These are: (a) Electrons, (b) Protons (c) Neutrons. The properties of these particles are summarized in the table below: Particle Electron Proton Neutron Position within the atom On the shell(s) or Orbit In the nucleus In the nucleus Charge Negative Positive Neutral Relative Mass 1/1840 or 0.00054 (negligible) 1 1 Note: The mass of an electron is so small compared to that of a proton or a neutron. So, it is usually ignored. The mass of a neutron is slightly higher that of a proton. The sum of the number of protons and neutrons account for the mass of an atom. Electronic configuration Atoms of different elements are of different sizes, with different numbers of protons, electrons and neutrons. The electrons in an atom revolve around the nucleus on paths referred to as orbits or shells. The number of shells allowable for an atom depends on the population of its electrons. The electrons are distributed into the different orbits following a set of rules. This distribution of electrons into cells is known as electronic configuration or electronic distribution or electron arrangement The rules are: (i) Electrons are placed into shell with lower energy before shells of higher energy. Energy of shells are denoted with values 1, 2, 3, 4, e.t.c. The shells are denoted with letters K, L, M, N, respectively. A “K” shell has a lower energy than a ‘L’ and would be filled before the ‘L’ shell. (ii) The K-shell requires a max. of 2 electrons to be stable, after which electrons begin to occupy the ‘L’ shell. The L-and M-shells require 8 electrons each to achieve stability though the maximum electrons that the M-shell can hold is 18, but it takes up electrons in two phases. More about the Electron Arrangement (Electronic Configuration) The electrons in an atom are arranged in shell(s) around the nucleus. For instance, Magnesium has 12 electrons, 12 protons and 12 neutrons; the electrons are arranged as follows: The electron arrangements for the first twenty elements are shown in the table below: Shorthand Representation of an Atom Atoms of an element can be described in a shot way as follow: Where: A (nucleon/mass number) = Number of proton(s) + Number of neutron(s). Z (Atomic number) = Number of proton(s) contained in the nucleus of an atom. So, Number of neutrons = A - Z Thus the atoms of Sodium and magnesium may be represented as follows: Sodium atom Magnesium atom Note: an atom is a neutral particle where the number of protons is equal to the number of electrons and be extension equal to the atomic number. CHEMICAL SYMBOLS A chemical symbol is a letter or a group of letters derived from the name of an element and used to represent the element. The following table shows the names of the first twenty elements, their chemical symbols and atomic number. Element Symbols Atomic Number Hydrogen H 1 Helium He 2 Lithium Li 3 Beryllium Be 4 Boron B 5 Carbon C 6 Nitrogen N 7 Oxygen O 8 Fluorine F 9 Neon Ne 10 Sodium Na 11 Magnesium Mg 12 Aluminium Al 13 Silicon Si 14 Phosphorus P 15 Sulphur S 16 Chlorine Cl 17 Argon Ar 18 Potassium K 19 Calcium Ca 20 Note that the symbols of an element may be derived from the: (i) first letter of its name in capital letter. E.g Carbon, Oxygen, Boron, Hydrogen, e.t.c. (ii) first two letters of its name. In such a case, the first letter is written in capital letter is in small letter. Examples are: Calcium, Ca; Aluminium, Al; Silicon, Si and Neon, Ne. (iii) first letter and another prominent letter in its name. Examples are Magnesium, Mg; Chlorine, Cl and Chromium, Cr. (iv) the latin name of the element. E.g. Sodium (or Natrium-Na) and Potassium (or Kalium-K). Other examples are: Element Latin name Symbol Iron Ferrum Fe Copper Cuprum Cu Gold Aurum Au Silver Argentum Ag Mercury Hydragyrum Hg Tin Stannous Sn Sodium Natrium Na Potassium Kalium K Atoms, Electrons, Electronic Configuration and the Periodic Table The table below shows the electron arrangement for the first 20 elements of the Periodic table. Notice how the shells fill up in order. Notes about the Periodic Table The elements are arranged in order of their proton number, row by row. The rows are called periods, and the columns are called groups. The period number shows how many shells there are. The group number for Groups I to VII is the same as the number of outer-shell electrons. ( the outer-shell electrons are often called the valence electrons) The outer-shell electrons of an element dictate how the element reacts. This is all the elements in Group I have similar reactions, because their atoms all have one outer-shell electron. The atoms of Group VIII elements (the noble gases) have 8 electrons in their outer-shells, except for helium, which has electrons 2. This arrangement makes these atoms stable. So the Group VIII elements are unreactive. . Molecule A molecule is a group of (two or more) atoms of either the same element or of different elements that can exist as a unit. For example, a molecule of oxygen (O2) contains two atoms of oxygen while a molecule of water (H2O) has two atoms of hydrogen but with an atom of oxygen. References Complete Chemistry for Cambridge Secondary I By Philippa Gardom Hulme https://youtu.be/LhveTGblGHY https://youtu.be/3_FJIpKgdV4 https://youtu.be/EMDrb2LqL7E Assignment: 1. Which of the particles in an atom: (a) has no charge? (b) has negligible mass? (c) is the heaviest? (d) is positively charged? 2. An atom of lithium has 3 protons. Its nucleon number is 7. (a) How many electrons does it have? (b) How many neutrons does it have? (c) What is the mass number, for lithium atoms? (e) Describe lithium atoms in the short way, using its symbol and numbers. 3. Name the element in: (a) Group VIII, Period 1. (b) Group V, Period 3. Mode of Submission: JS 2 Beta and 2 Chi Students should submit to Mr Osadare via: osadarephilip20@gmail.com JS 2 Alpha and 2 Delta Students should submit to Mr Olasupo via: abdulqadrola@gmail.com