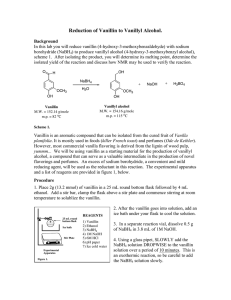
THE UNIVERSITY OF THE WEST INDIES Department of Chemistry ST. AUGUSTINE CAMPUS ST. AUGUSTINE, TRINIDAD & TOBAGO, WEST INDIES NAME: JEREMIAH NATHANIEL MATHURA COURSE: CHEM 3670 ACADEMIC YEAR: 2021/2022 SEMESTER: 1 SUPERVISOR: DR. RAMISH PINGAL TITLE: IMPROVING THE FRAGRANCE QUALITY OF VANILLIN TABLE OF CONTENTS Abstract…………………………………………………………………… 3 Introduction & Literature Review…………………………………………4 Objectives………………………………………………………………….8 Experimental Methodology………………………………………………..10 Analysis……………………………………………………………….…...11 Discussion…………………………………………………………………19 Challenges & Limitations……………………………...………………….24 Recommendations…………………………...……………………………24 Conclusion………………………………………………………………..25 References……………………………………………………………......26 ABSTRACT The fragrance industry makes up a significant proportion of the Global Market and producing a cost-effective fragrance or flavor requires investigating the type and proportion of ingredients that ends up in the final product. Volatile organic molecules are important constituents in several fragrances and flavors that are available on the buyer’s market. Vanillin is a very essential aromatic compound that is used in the fragrance industry. Vanillin is used in the food and beverage industry, in perfumes and colognes and certain isomers of vanillin has medicinal properties. Vanillin can be synthesized artificially in the laboratory from starting products such as guaiacol, eugenol and 4-hydroxybenzaldehyde. Of equal importance, is the fact that vanillin can be produced naturally, through the benzoate and ferulate acid biosynthetic pathways. The artificial synthesis of vanillin and its derivatives becomes very important, because constant extraction of natural vanillin is unfeasible. This is due to some species are either rare or protected by law. It is also impossible to meet the large demand for vanillin, by use of extraction. Synthesizing Vanillin artificially in the lab is cost-effective and allows for the possibility of an ideal Vanillin molecule to be synthesized. Hence this research is geared towards looking at different derivatives vanillin molecules, by investigating which functional groups are responsible for the physical properties of vanillin, such as smell. This research demonstrated that four vanillin derivatives can be synthesized and they all have ester groups, along with aldehyde and alkoxy groups, in its structure. These proposed structures are expected to give an amplified odour, when compared to vanillin. The ester groups would give the compound a floral/fruity smell, which can be desirable to the consumer. In this research, all the proposed molecules had a larger relative molecular mass (RMM) than that of the isomers of vanillin. The greater the RMM, the lower the volatility of the substance. The scent may have been improved, but the volatility has been reduced. INTRODUCTION Organic fragrances are very important constituents of perfumes, colognes, air fresheners, soaps, beverages and in detergents. Whilst we are familiar with several pleasant smells that are either citrus, fruity, sweet, minty, or fragrant, there are several pungent smelling sensations that we get, when something is smelling repulsive1. The brain in conjunction with our olfactory system, within our nostrils can differentiate between a plethora of scents and odours. Therefore, it is important to gain a comprehensive understanding about the chemical and physical properties of the molecules whose scent is detected. The molecules that are detected, are small, volatile organic molecules that can travel easily. Investigating the structure and functional group that elucidates a particular smell, would aid us in determining what types of organic compounds can be synthesized artificially, when making fragrances1. Once a clear understanding of the relationship between the functional group and structure and the olfactory responses, then cost effective methods used for synthesizing these compounds, can be investigated2. This study shall delve into the chemistry of fragrances and how the structure of the organic molecule interacts with the olfactory receptors in our noses, to formulate a certain smell. There are several classes of organic molecules such as esters, terpenes, amines, aromatics, aldehydes, ketones, and lactones3. Certain organic molecules are shaped in a way that they can fit into a specific receptor cell, thus allowing the brain to detect a certain smell. Studying fragrances and the compounds that constitutes these fragrances are very important. Studying these compounds and looking for cheaper cost-effective alternatives of fragrances to be used as perfumes, colognes, and air fresheners. In a time in Trinidad and Tobago, where there is an economic decline in the Oil and Gas Industry, it is no secret that we as a nation need to find other economic avenues to diversify our Economy. Our country is home to a lot of tropical plants that contains several compounds that can be used to make fragrances. Another important angle of this research requires us to recognize the economic benefits of repulsive and pungent odours that emanate from garbage, rotten meat, spoilt food, and faeces. When these unpleasant odours emanate, there is the immediate desire, for the person who is smelling that odor, to try to neutralize these odours with something that is pleasant smelling4. This is the reason why there has many so much extensive research on organic compounds that would be suitable to be included in air fresheners. The same principle is used to explain the economic benefits of perfumes, colognes, deodorants, and other products that utilizes fragrances, to ensure that the user emanates a pleasant smell. Certain scents are naturally occurring, and others are artificial, created experimentally. There are several naturally occurring organic compounds that are used in fragrances, such as vanilla and limonene5. However, utilizing naturally occurring fragrances, for mass manufacturing of these products that utilizes these fragrances, may be cost ineffective. As a result, Chemists have opted to manufacture, cheaper artificial fragrances, which are similar in smell of these naturally occurring scents6. Therefore, it is most noteworthy to study the structure of the different class of Organic molecules and monitor their respective shapes, to ascertain the scent these molecules would give off7. Carefully analyzing organic molecules that are created synthetically, would be useful in determining whether the compound can be used to produce the desired scent. It is also more environmentally friendly to use synthetic fragrances as opposed to natural fragrances, resulting in the conservation of our local fauna. Additionally, studying and analyzing the structure and identity of organic compounds can be done using several spectroscopic techniques such as Nuclear Magnetic Resonance Spectroscopy (NMR) and Infrared Spectroscopy (IR) and mass spectrometry8. These techniques will enable us to perform structural elucidation on several unknown compounds and when this is done, these compounds can be compared to a known family of compounds that emanate a certain scent or odour. The merits of research into fragrance chemistry would enable us to preserve the environment, and this project also has potential for economic diversification9. Research in this field would always be beneficial, because of the constant need to eradicate foul odours. As the saying goes, “necessity is the mother of all invention. The eradication of foul and unpleasant odours would always be a need. If in Trinidad and Tobago, a unique, high quality and cost-effective alternative to several of our imported fragrance – based products, is produced, this would have the effect of unlocking our Nation’s potential for economic diversification9. If Trinidad and Tobago can reduce its imports of fragrance – based products, and coincidentally increase their exports, the potential of economic growth, becomes closer to a reality. A lot of fragrances use vanillin which is extracted from the vanilla beans of vanilla orchids. There are over 25,000 species of vanilla orchids around the world. These vanilla orchids are native to South and Central America, as well as in the Caribbean. Trinidad and Tobago have a few species of orchids10. Vanilla is the second most expensive spice in the world, but its importance and value as a constituent of several fragrances, cannot be underscored. Vanillin had a market size value in 2019, of 480.1 million USD & Revenue forecast in 2025, of 734.5 million USD, with a growth rate of 7.1% for this period. Vanillin can be synthesized artificially using guaiacol, eugenol and lignin as starting compounds4. This research intends to narrow its focus on natural vanillin species that are found right here in Trinidad and Tobago and explore the potential to synthesize cheaper artificial alternatives of these compounds. Vanilla is an example of a base note that is used in fragrances and base notes add to the long-lasting scent of fragrances5. The top and heart notes of fragrances are detected by the user before the base note is detected and these notes last for a shorter period. It is economically difficult for Trinidad and Tobago to commercially manufacture an entire fragrance for exporting. However, it would be prudent to explore compounds that can be used as constituents of the fragrance. Seeing that Trinidad and Tobago is home to many species of vanilla including Vanilla Hartii, Vanilla Inodora and Vanilla Gradifora.10 Therefore, there is potential for Trinidad and Tobago to become contributors in the fragrance making industry. However, it would be more sustainable for artificial substitutions of the vanillin compound in these species to be synthesized because extraction of the vanillin compounds from vanilla orchids, which are either rare or protected is economically unfeasible. In this research, the structural properties of different vanillin substitutes, would be analyzed using Nuclear magnetic Resonance (NMR) and Infrared (IR) spectroscopy11. These spectroscopic techniques would be employed for us to make comparison between vanillin derivatives, synthesized artificially and vanillin extracted from locally found vanilla orchids. It is imperative that there is a clear understand the structure of vanillin because as mentioned earlier, the structure of these organic molecules determines the type of odour that will be detected11. In this research laboratory synthesis techniques would be explored and cost-effective means of synthesis of these vanillin derivatives would be investigated. Ethyl vanillin is one example of a vanillin derivative and has a scent that is much stronger than vanillin11. Apart from synthesizing these vanillin derivatives, the structures should be carefully examined. Some vanillin derivatives that give of stronger scents would interact with the olfactory system differently when compared to regular vanillin. According to the cited literature, it has been shown that the scent of all vanillin species would not have the same exact intensity9. Hence, research into the intensity of different forms of vanillin is worth investigating. Furthermore, based on research previously done, it was discovered that in vanilla orchids there are chemical reactions that involve the production of primary metabolites which are used for growth and secondary metabolites is not used for growth, reproduction, and development of the plant species. Secondary metabolites and their respective bioactivities found in the native vanilla orchids found in Trinidad & Tobago, can have several medicinal and health benefits11. The properties of these secondary metabolites are worth investigating as there is economic potential in the field of Pharmaceutical Chemistry. OBJECTIVES Based on the reviewed literature, the following objectives would be investigated in this research project: • Improve the fragrance quality of vanillin used in the Fragrance Industry. • Look into alternative ways to synthesize vanillin derivatives in order to reduce the need for natural extraction of vanillin. • Improve the quality of odours by devising a vanillin molecule that would emanate a more potent smell, which then is included in the ingredients for a fragrance that contains vanillin. • Determine how the presence or absence of certain functional groups on vanillin will affect its odour. • Investigate potential designer molecules for vanillin, which have not already been synthesized and is not available on the buyer’s market. • Illustrate some biosynthetic pathways in which vanillin is synthesized In summary, previous research indicates the potential for economic diversification as fragrances will always have economic value, due to its importance in eliminating foul odours. Vanillin, being one of the most popular base notes and constituents of several fragrances, there would be high demand for this compound. However, due to some vanilla orchids being rare or classified as a protected species, there is need for the synthesis of artificial derivatives. These derivatives and substitutes for naturally occurring vanillin, would have a similar structure resembling that of natural vanillin. Therefore, the mode of action of these vanillin substitutes, would be very similar, with slight variations in the strength of the scent, perceived by the consumer. EXPERIMENTAL METHODOLOGY In this research, it was decided that there would be no practical components implored, due to the restrictions brought on by the COVID-19 Pandemic. The following methodology would be implored: 1. We would be looking at the methods in which vanillin can be synthesized from: i. Eugenol ii. Guaiacol iii. 4-Hydroxybenzaldehyde 2. We would be looking at vanillin derivatives such as Isovanillin, o-vanillin, ethyl vanillin and looking at what odours they emanate and we would check to see it the odour is stronger than vanillin. 3. In this research we shall look at how smell is detected and perceived by the olfactory system and use this theory to justify altering the functional groups and positions of these functional groups, in our proposed designs. 4. We would propose a reaction pathways for four possible alternatives of vanillin 5. In this research we will illustrate several biosynthetic pathways that vanillin can be produced and have a brief discussion on their significance and potential for further research. ANALYSIS The structure of vanillin contains a methoxy, a hydroxyl and a benzaldehyde group on it. These functional groups is what gives vanillin its characteristic scent. It must be noted that an absence of any functional group could result in a totally different scent, being emanated. Additionally the presence of multiple of the same functional groups can result in an amplification of the scent. All of these functional groups play an important role in how the scent is perceived by the brain. Analyzing the effects of these functional groups would enable us to form a designer molecule similar to vanillin and can be a suitable replacement to vanillin. An ideal designer molecule would be cheaper to produce, have an amplified pleasant smell and is not toxic or harmful to the consumer in any way. One interesting point to note is the presence of the aldehyde functional group which is an integral component of benzaldehyde, which has a pleasant smell. Another important thing to note is the presence of the hydroxyl group which gives room for esterification which enables the formation of an ester, which is pleasant smelling. Figure 1.0 showing the structure of vanillin Figure 1.1 showing the NMR Spectra for Vanillin Figure 1.2 showing the assignment of peaks for vanillin Organic Compound Smell Emanated Benzaldehyde strong sharp sweet bitter almond fruity cherry maraschino cherry cherry oily nutty woody tropical fruit sweet, acrid odor (like plastic N/A rubber) aromatic, refreshing and sweet aromatic acrylate acetone (herbal) plastic Phenol Anisole Flavour Veratraldehyde sweet woody vanilla Ethyl Vanillin Pleasant odor (stronger than vanillin) Faint sweet vanillin odour Vanillin taste Acetovanillone Vanillin Acetate Vanillin Isobutyrate sweet creamy vanilla-like sweet, creamy, vanilla, Sweet, vanilla, creamy, powdery, heliotropin odour powdery with a balsamic beany nuance sweet vanilla creamy fruity sweet vanilla powdery caramellic chocolate chocolate creamy buttery fruity cheesy Table 1.0 showing the smells emanated for organic compounds, possessing only one functional group that is found in vanillin. From the table above, we see that having the hydroxyl group present on the ring as seen in phenol, does not give a pleasant smelling compound. Hence, the effects of this functional group should not be amplified, in the creation of the designer vanillin molecule. The ideal molecule would in the form of an ester with the methoxy and benzaldehyde group, still being present. As seen with vanillin acetate and vanillin isobutyrate, the pleasant scent has been amplified when vanillin is esterified. In order for us to propose a reaction synthesis of our designer molecule we would first delve into the reaction synthesis techniques involved in the formation of regular vanillin for various starting products. Figure 2.0 showing vanillin synthesis from Eugenol Glyoxylic acid Guaiacol Vanillin Figure 3.0 showing vanillin synthesis from Guaiacol Figure 4.0 showing vanillin synthesis from 4-hydroxybenzaldehyde We have looked at various ways these functional groups can be added, to form vanillin. However these functional groups can be added in a different arrangement on the benzene ring. Two examples of this can be seen in: 1) O-Vanillin 2) Isovanillin In o-vanillin, the hydroxyl group is no longer para to aldehyde group, but instead it is ortho to the benzaldehyde group, the methoxy group is still meta to the aldehyde group, as seen in vanillin. In Isovanillin, the methoxy groups is para to the aldehyde group and the hydroxyl group is meta to the aldehyde group. Whilst these two isomers can be purchased by a chemical supplier, there are several derivatives of these vanillin isomers that have not been explored. LOOKING AT THE BIOSYNTHETIC PATHWAY AT WHICH VANILLIN IS PRODUCED Figure 5.0 showing Vanillin being synthesized by the Benzoate and Ferulate Biosynthesis pathways, with phenylalanine as the starting product. Figure 6.0 showing multiple biosynthetic pathways in which vanillin is formed with the respective coenzymes and processes involved5 Figure 7.0 showing the biosynthetic conversion of eugenol to vanillin5 SYNTHESIZING THE IDEAL VANILLIN DERIVATIVE When designing the proposed ideal vanillin derivatives the following considerations should be made: • Type of functional groups present on the benzene ring, certain functional groups contribute to the smell that is emanated • Isomers of the same molecule would elicit a different smell, for example, o-vanillin and Isovanillin have a stronger odor than regular vanillin • The Relative Molecular Mass of the compound is important, as the smaller the molecule, the more volatile it becomes more readily vaporized and can travel alongside air molecules and into the nostrils, where the scent can be detected. • Cost incurred for the synthesis of this designer molecule. PROPOSED STRUCTURES AND SYNTHESIS PATHWAYS We were able to propose 4 proposed designer molecules and propose stepwise synthetic pathways for each proposed structure. DISCUSSION From what we would have done, it was demonstrated that there are method in which we can synthesize four compound that would be expected to give of a similar but stronger odour than vanillin. Seeing that in all proposed molecules the hydroxyl group was esterified, we can expect the compound to emanate a fruity/floral odour, which is characteristic of esters. We would have kept the alkoxy and benzaldehyde functional groups on each of the proposed set of molecules. However, before we go deeper into the rationale for choosing these prospective designer molecules, let us first discuss how the structure of these organic molecules are related to the activity of the olfactory senses. When an odour reaches the nose, there is a biological aspect that explains how it is detected: • Volatile odour molecules enter the nose, head up the nostrils, and then proceeds into the nasal cavity. • In the nasal cavity, the odour molecules reach a postage stamp-sized area at the top of this space called the olfactory epithelium. This region consists of bundles of neurons containing olfactory receptors. • The receptors bind with the molecules and send signals to the brain, which then interprets the odour, and links it a particular smell which may be pleasant or unpleasant. The mode of action as described above gives a surface understanding of how these odours are detected, however it does not give an insight on how exactly the molecules are interact with the olfactory receptors. There are three underlying theories that gives us an insight on how this occurs: 1. Lock and Key5 – This model is similar to the inductive fit model and it suggests that odour molecules would gave different shapes and these odour molecules would be compatible with each of their respective olfactory receptors, similar to how a key fits into a lock. The odour molecules each with a different shape and size would now be fitted into a different receptor, which is compatible with. The molecule or receptor can alter its shape to enable an induced fit. When a molecule locks into a receptor, the receptor now transmits signals to the brain, thus allowing us to perceive the odour of the substance. Consequently we can assume that there is one receptor for each odour that is detect by the person smelling. 2. Vibrational Theory5 – There is an alternative model that involves the principles of Physics. This model claims that the olfactory receptors detect these odour molecules based on the frequencies at which these bonds vibrate. Each molecular bond possesses a specific resonant frequency at which this bond naturally vibrates. Different molecules would possess a unique set of vibrational frequencies, depending on which atoms the molecule consists and the arrangement and connection of the atoms of these bonds. 3. Quantum Tunneling5 – This theory is very similar to the vibrational model and it suggests that the odour of a molecule is dependent vibrational frequency of said molecule, so if you could alter the frequencies of the molecule, you can also alter its smell. Our organoleptic smell receptors wait for an odor molecule to come in that allows an electrons of the molecule to tunnel across the receptor, and trigger the particular nerve in the olfactory receptor corresponding to that energy. Now that an explanation on the principles of odour perception and organoleptic properties has been rendered, we can now relate this theory to the rationale for exploring different vanillin derivatives. When looking at different vanillin derivatives, we notice that there is always an aldehyde group and an aromatic benzene ring. According to the theory, we can say that these functional groups have bonds that vibrate at different frequencies, causing certain receptors to trigger a response to the brain. Figure 8.0 Showing the Olfactory System5 In each of the proposed molecules we can see that it contained and ester group, replacing the hydroxyl group. The rationale behind this is the antiseptic odour given off by phenolic compounds. The ester group would provide a more floral or fruity odour and this would be more pleasant to the consumer. We would have made the decision to maintain the presence of the aldehyde functional and alkoxy functional groups, because both groups are important in attaining the desired odour. It was important that the ester and methoxy groups are kept to a maximum of 3 carbons, this is because, we would have found out that as the carbon chain increases, this results in the compound emanating an unpleasant odour. So we not only want to keep the volatility as high as possible, by reducing our relative molecular mass, we also do not want an odour that is unpleasant to the consumer. Now we can discuss the previously mentioned theory and relate it to our own proposed structures. When we chose to change the types of bonds and the order of these bond, we are thereby altering the interactions between the molecule and the receptors. If we were to consider the lock and key model or the induced fit model, it is safe to assume, that the receptor molecule would have to adjust its shape for the molecule to fit or an entirely different receptor would detect the proposed molecule. If were we to consider the bond vibration frequencies as discussed in the vibrational and quantum tunneling theories, we can clearly see that the bond frequencies would have been altered. In the case where we have two aldehyde groups in the structure, we have a situation, where there can be expected amplification of the signals that are going to the brain. Replacing the hydroxyl group with an ester functional group would also have alterations in the frequencies that are detected by the receptors and this would result in different signals being sent to the brain. The underlying principle of this study is that changing the bonds and functional groups present on the aromatic ring, results in different types of receptors being triggered and therefore a different signal would be produced, leading to an amplification of the scent. Since we would have already discussed in great detail the results of the research, we shall briefly look into the secondary metabolites produced in the biosynthesis of vanillin. We see that in the biosynthetic pathway several biological enzymes and energy stored in the form of ATP, are involved. In these biosynthetic pathways, several of the byproducts such as coniferyl and guaiacylglycerol are involved in other biosynthetic routes leading to a cascade of other biological products that can now be used for other biological functions. These secondary metabolites provide opportunities for the development of several medicinal drug, provided that research is conducted. CHALLENGES AND LIMITATIONS This synthesis of alternative derivatives of vanillin comes with the following potential challenges and limitations: • Whilst the addition of certain functional groups to vanillin isomers, can potentially enhance the scent and flavor emanated, all the proposed molecules had an increased relative molecular mass (RMM), thus reducing volatility. • The synthesis of these molecules may end up being costly as the actual synthesis of these molecules requires optimal conditions, which may be costly to maintain. • Due to the Covid-19 restrictions, no laboratory or practical work was done. • Due to the lack of the practical component of this research, the actual yields for these proposed derivatives, could not have been determine. The inability to determine the yield, affords us little potential in improving the actual practical method of synthesis. • Little research has been done on the physical properties of o-vanillin, as it is more widely used for medical purposes, as opposed to the fragrance and flavor industry. RECOMMENDATIONS Apart from carrying out a practical synthesis of these compounds and experimentally determining the yield, it would be useful to compare the product yield of the synthesis of these four proposed molecules, with the yield obtain from extraction of natural vanillin. This would allow us to determine whether the synthesis of these proposed molecules, would serve as a viable alternative for vanilla extraction. CONCLUSION From the research conducted, several ways vanillin can be synthesized, with several different starting products such as eugenol, guaiacol and 4-hydroxybenzaldehyde. Vanillin can also be synthesized via biosynthetic pathways in vanilla orchids, the intermediate by products of these pathways, have several medicinal benefits. Synthesizing vanillin artificially, can lower the production costs associated with extraction of vanillin and artificial vanillin synthesis also allows for conservation of endemic vanillin species. Artificial vanillin production is likely to produce a higher yield than extraction and this is economically beneficial. Four designer vanillin derivatives, were proposed by looking at isomers and functional groups of vanillin which could result in a molecule that would cause the emanation of a more potent odour, desirable to the consumer. REFERENCES 1. David Pybus, C. S., The chemistry of fragrances. 1999, 51-79. 2. Parry, E. J., The chemistry of essential oils and artificial perfumes. 1st ed.; Scholar Select: London, 1918; Vol. 1. 3. The Chemistry of Fragrances_ From Perfumer to Consumer. In Chemistry of Fragrances, Sell, C., Ed. RSC Publishing: 2006; pp 229-252. 4. Sharmeen, J. B.; Mahomoodally, F. M., Zengin, G.,; Maggi, F., The Essence's of Perfume Materials - Fragrance Ingredients. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals 2021. 5. Allen, C.; Havlíček, J.; Roberts, S. C., The Effects of Artificial Fragrances on Human Olfactory Communication. 2019, 107-117. 6. Fráter, G.; Bajgrowicz, J. A.; Kraft, P., Fragrance chemistry. In Tetrahedron, 1998; Vol. 54, pp 7633-7703. 7. Industry, T. I. F. A. I. O. o. t. F., <IFRA-IOFI-DSI.pdf>. In Comments on the potential implications of the use of Digital Sequence Information (DSI) on the objectives of the Nagoya Protocol (NP), 2017. 8. Chemistry and Technology of Flavours and Fragrances. Rowe, D. J., Ed. Blackwell Publishers: United Kingdom, 2005; pp 305-330. 9. Berger, R. G., Flavours and Fragrances_ Chemistry, Bioprocessing and Sustainability Springer: Germany, 2007. 10. SCHULTES, R. E., Native Orchids of Trinidad and Tobago. In Native Orchids of Trinidad and Tobago, Elsevier Science: London, 1960; Vol. 3, pp 39-44. 11. Belange, D. H. F. a. F. C., Handbook of Vanilla Science and Technology. Second Edition ed.; John Wiley & Sons Ltd.: United Kingdom, 2019; p 527.