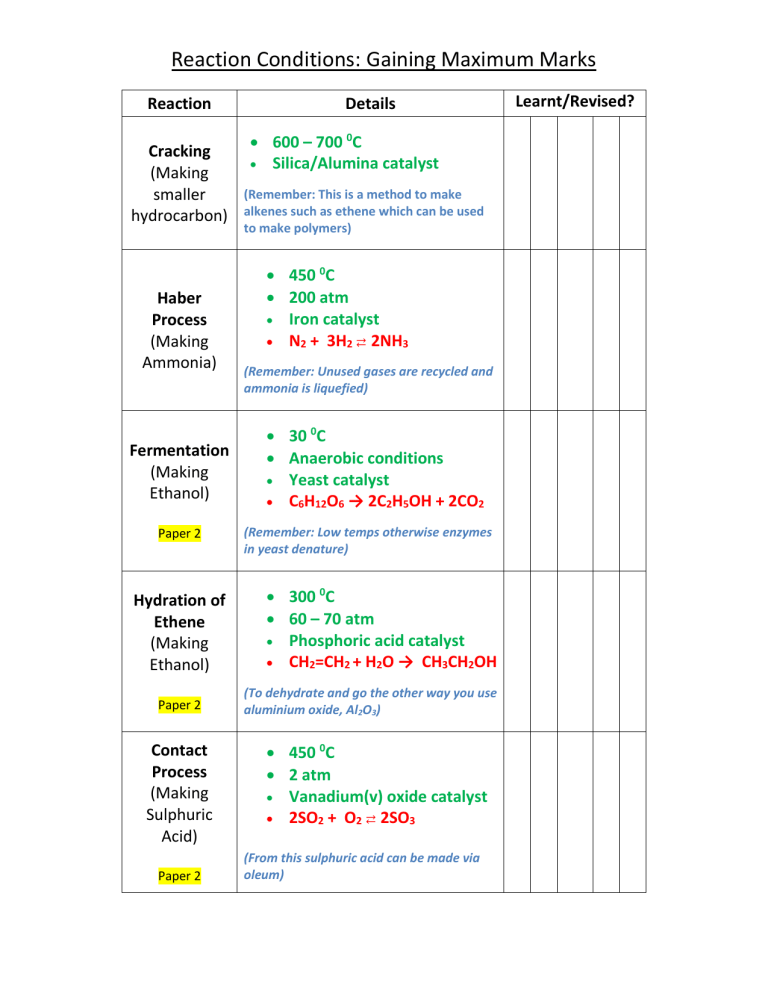
Reaction Conditions: Gaining Maximum Marks Reaction Cracking (Making smaller hydrocarbon) Haber Process (Making Ammonia) Fermentation (Making Ethanol) Paper 2 Hydration of Ethene (Making Ethanol) Paper 2 Contact Process (Making Sulphuric Acid) Paper 2 Details 600 – 700 0C Silica/Alumina catalyst (Remember: This is a method to make alkenes such as ethene which can be used to make polymers) 450 0C 200 atm Iron catalyst N2 + 3H2 ⇄ 2NH3 (Remember: Unused gases are recycled and ammonia is liquefied) 30 0C Anaerobic conditions Yeast catalyst C6H12O6 → 2C2H5OH + 2CO2 (Remember: Low temps otherwise enzymes in yeast denature) 300 0C 60 – 70 atm Phosphoric acid catalyst CH2=CH2 + H2O → CH3CH2OH (To dehydrate and go the other way you use aluminium oxide, Al2O3) 450 0C 2 atm Vanadium(v) oxide catalyst 2SO2 + O2 ⇄ 2SO3 (From this sulphuric acid can be made via oleum) Learnt/Revised?



