AP Chemistry Catalysts
advertisement

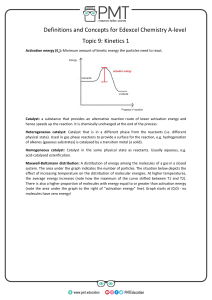
Name______________________________________________Pd_____Date____________ AP Chemistry Catalysts Things to know: A catalyst . . . - Is a substance that increases the rate of the reaction without being consumed. - Speeds up both the forward and reverse reactions by: o Making the activation energies lower o Making the rate constant higher - Does not yield more product, but yields product more quickly. - Provides a different mechanism for the reaction to occur (lowering the energy pathway. - Consists of two types: o Homogeneous catalyst – exists in solution with the reaction mixture o Heterogeneous catalyst speeds up a reaction that occurs in a separate phase most often a solid interacting with gaseous or liquid reactants needs large surface area to be effective Example: Metal-catalyzed hydrogenation of hydrocarbons. (fig 15-24 book)