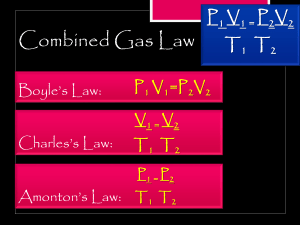
Name:___________________________________________Grade & Section:________________ BOYLE’S LAW QUIZ Boyle’s law states that at a constant temperature, the pressure of a gas is inversely proportional to its volume. 1. An ideal gas occupying a 2.0 L flask at 760 torrs is allowed to expand to a volume of 6,000 mL. Calculate the final pressure. Given: Formula: Solution: Final Answer: 2. A gas occupies a volume of 1 L and exerts a pressure of 400 kPa on the walls of its container. What would be the pressure exerted by the gas if it is completely transferred into a new container having a volume of 3 litres (assuming that the temperature and amount of the gas remain the same.)? Given: Formula: Solution: Final Answer: 3. A gas exerts a pressure of 3 kPa on the walls of container 1. When container one is emptied into a 10 litre container, the pressure exerted by the gas increases to 6 kPa. Find the volume of container 1. Assume that the temperature and amount of the gas remain the same. Given: Formula: Solution: Final Answer: 4. A gas is initially in a 5 L piston with a pressure of 1 atm. What is the new volume if the pressure changes to 3.5 atm by moving the piston down? Given: Formula: Solution: Final Answer: 5. A gas in a 30.0 mL container is at a pressure of 1.05 atm and is compressed to a volume of 15.0 mL. What is the new pressure of the container? Given: Formula: Solution: Final Answer: Name:___________________________________________Grade & Section:________________ CHARLES’ LAW QUIZ Charle’s law states that at constant pressure, the volume of a gas is directly proportional to the temperature. 1. A gas occupies a volume of 600.0 mL at a temperature of 20.0 °C. What will be its volume at 60.0°C? Given: Formula: Solution: Final Answer: 2. A gas occupies a volume of 900.0 mL at a temperature of 27.0 °C. What is the volume at 132.0 °C? Given: Formula: Solution: Final Answer: 3. What change in volume results if 10.0 mL of gas is cooled from 33.0 °C to 15.0 °C? Given: Formula: Solution: Final Answer: 4. A gas occupies a volume of 1 L at a temperature of 17.0 °C. What is the volume at 10.0 °C? Given: Formula: Solution: Final Answer: 5. A gas occupies a volume of 500.0 mL at a temperature of 10.0 °C. What will be its volume at 50.0 °C? Given: Formula: Solution: Final Answer: