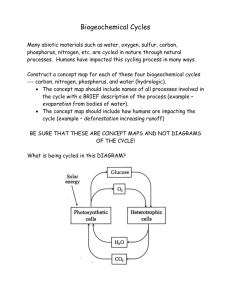
Chapter One Environmental Chemistry and Toxicology 1. Introduction to Environmental Chemistry Chemistry is defined as the science of matter. Therefore, it deals with the air we breathe, the water we drink, the soil that grows our food, and vital life substances and processes. Our own bodies contain a vast variety of chemical substances and are tremendously sophisticated chemical factories that carry out an incredible number of complex chemical processes. Environment is the place where we live or work, the atmosphere which we breathe and water which we drink, unspoilt areas of the world which could soon be ruined, and part of the atmosphere which shields us from harmful radiation. Environmental chemistry is the branch of chemistry that deals with the origins, transport, reactions, effects, and fates of chemical species in the water, air, earth, and living environments and the influence of human activities thereon. It overlaps with different branches of chemistry such as organic chemistry, analytical chemistry, physical chemistry, photochemistry, geochemistry and biological chemistry and also includes many widely different fields such as physics, life sciences, agricultural sciences, medical sciences, public health and sanitary engineering. For convenience our environment can be divided into the atmosphere, the geosphere, the hydrosphere, the biosphere, the anthroposphere, and all the fauna and flora. The terms fauna and flora are collective names given to animals and plants respectively. There is a continuous interaction between the various sections of the environment and the flora and fauna. An assembly of mutually interacting organisms and their environment in which materials are interchanged in a largely cyclical manner is known as ecosystem. The environment in which a particular organism lives is called habitat. Ecology is the science that deals with the relationships between living organisms with their physical environment & with each other. An ecosystem consists of an assembly of mutually interacting organisms and their environment in which materials are interchanged in a largely cyclical manner. Introduction to Environmental Chemistry Page 1 Environmental science in its broadest sense is the science of the complex interactions that occur among the terrestrial, atmospheric, aquatic, living, and anthropological environments. For our purpose, environmental science is defined as the study of the earth, air, water, and the living environments, and the effects of technology there on. It includes such disciplines, as chemistry, biology, ecology, sociology, & government that affect or describe these interactions. A related discipline, toxicological chemistry, is the chemistry of toxic substances with emphasis upon their interaction with biological tissue and living systems. Besides its being an essential, vital discipline in its own right, environmental chemistry provides an excellent framework for the study of chemistry, dealing with “general chemistry,” organic chemistry, chemical analysis, physical chemistry, photochemistry, geochemistry, and biological chemistry. Environmental chemistry encompasses many diverse topics. It may involve a study of Freon reactions in the stratosphere or an analysis of PCB deposits in ocean sediments. It also covers the chemistry and biochemistry of volatile and soluble organometallic compounds biosynthesized by anaerobic bacteria. Literally thousands of other examples of environmental chemical phenomena could be given. Chemists must be aware of the possible effects of their products and processes might have upon the environment. Furthermore, any serious attempt to solve environmental problems must involve the extensive use of chemicals and chemical processes. There are some things that environmental chemistry is not. It is not just the same old chemistry with a different cover and title. Because it deals with natural systems, it is more complicated and difficult than “pure” chemistry. Students sometimes find this hard to grasp, and some traditionalist faculty find it impossible. Accustomed to the clear-cut concepts of relatively simple, well-defined, though often unrealistic systems, they may find environmental chemistry to be poorly delineated, vague, and confusing. More often than not, it is impossible to come up with a simple answer to an environmental chemistry problem. But, building on an ever-increasing body of knowledge, the environmental chemist can make educated guesses as to how environmental systems will behave. Introduction to Environmental Chemistry Page 2 1.2. Components of Environmental Chemistry The environmental chemistry comprises the following components: A) Aquatic Chemistry ➢ The study of water in rivers, oceans, lakes, ensuries, underground water and the phenomena that determines the distribution, circulation of chemical species in natural water. B) Atmospheric Chemistry ➢ Deals with the chemical species that determines the general physical and chemical properties of the surrounding atmosphere C) Soil Chemistry ➢ The field of study about the chemical and physical processes that occur in the soil matrix. D) Green Chemistry ➢ Technologies of the invention, design and application of chemical products and processes to reduce or to eliminate the use and generation of hazardous substances, and where possible utilize renewable raw materials Introduction to Environmental Chemistry Page 3 E) Toxicological chemistry ➢ Is the chemistry of toxic substances with emphasis upon their interaction with biologic tissue and living systems. 1.3. Properties of Environmental Chemical Species A very wide range of chemicals species present each sphere of environment. These chemicals broadly classified into: A) Chemical that causes human toxicity There are many chemicals which affect typical human being and pose serious effect. Some chemicals such as Pb, Cd and Hg are well known for their adverse effects of human health at high level of exposure. Have no essential role in human body i.e based on exposure these metals are considered non- essential. Environmental exposure of these chemicals cause cancer; especially benzene which release from the vehicle emission and polycyclic aromatic hydrocarbon and chlorinated dioxin from combustion of fossil fuel. B) Chemical which causes damage to non-human biota but are not believed to human at current level of exposure Many elements and compounds are categorized in this group. They affect plants and animals seriously and indirectly can affect human health. Example, Cu and Zn are essential trace element for human and environmental exposures very rarely present at risk to health. Toxic to plant growth at high level. Polychlorinated biphenyl disrupts the reproduction and growth of wild life species. C) Chemicals not directly toxic to human These are not toxic other biota at current environmental concentrations but capable of causing environmental damage. The prime example is chlorofluorocarbon (CFCs), which is found wide spread to the environment and causing major disruption to the chemistry of stratosphere. Introduction to Environmental Chemistry Page 4 1.4. Environmental Transformation and Degradation The process in which a substance in the environment is modified by biological or non- biological mechanism is known as transformation. Degradation usually refers to the breakdown of the original molecule by the loss of the various component parts or by the fragmentation of the molecule into smaller substances. This is partial change in the structure and amount of contamination due to biological/non biological reaction i.e biotic and abiotic transformation. The mechanism of decreasing the concentration of contaminant due to reversible alteration of chemical structure. Both transformation and degradation either biotic or abiotic are environmental attenuation process. The attenuation process is transformation of parent molecule to either toxic or non-toxic product form. Reduction in concentration. Changing the structure of parent molecule Inactivation (detoxification) i.e loss of toxic effect. Activation (toxification) pose chemicals become toxic by changing the structure. Mineralization i.e releasing of essential nutrients as a result of decomposition of large molecules by biotic/abiotic degradation. The process of biodegradation: Bacteria cell containing enzymes take up chemicals. Chemicals bind to suitable enzyme (enzyme-complex). Enzyme chemical-complex reacts, producing transformation product. Products released from enzyme. Sorption in the soil many influence in the process above. Production of new or additional enzymes capacity. Growth of total microbial population and thus biodegradation capacity. Introduction to Environmental Chemistry Page 5 Abiotic transformation a) Hydrolysis - addition of water to a molecule (usu. to functional groups) - often enhanced by the presence of acids or bases b) Redox reaction - promoted by low conc. of oxygen radicals, mineral surfaces, UV/ozone/Fenton's reagent/TiO2 (collectively called AOP)/Fe0... c) Photolysis - light-induced redox reaction - chemicals must be on surface or where light can reach Biotic transformation a) Biodegradation vs. mineralization b) The most significant removal pathway of contaminants from natural environment. 1.5. Matter and cycles of matter Matter is anything that occupies space and has rest mass (or invariant mass). It is a general term for the substance of which all physical objects consist. Typically, matter includes atoms and other articles which have mass. Mass is said by some to be the amount of matter in an object and volume is the amount of space occupied by an object, but this definition confuses mass and matter, which are not the same. Cycles are sequences of reoccurring events. Cycles of matter, often based on elemental cycles, are utmost importance in the environment. Organisms participate in biogeochemical cycles, which describe the circulation of matter, particularly plant and animal nutrients, through ecosystems. The flow are delicately and naturally balanced (no excess-no deficiency). Biogeochemical Cycles Biogeochemical cycles are ultimately powered by solar energy. These cycles interchange matter among the atmosphere, biosphere, anthroposphere, geosphere, and hydrosphere. Cycles can be divided as Endogenic and Exogenic. Endogenic cycles are types of cycles, which predominantly involve subsurface rocks of various kinds, and exogenic cycles, which occur largely on Earth‘s surface and usually have an atmospheric component. Most biogeochemical cycles can be described Introduction to Environmental Chemistry Page 6 as elemental cycles involving nutrient elements such as carbon, nitrogen, oxygen, phosphorus, and sulfur. The water and chemical elements that organisms need continuously cycle through ecosystems, passing repeatedly through their biotic and abiotic components. These cycles are called biogeochemical cycles, because they are cycles of chemicals that include both organisms (bio) and abiotic components, such as the ocean or rocks (geo). As matter moves through a biogeochemical cycle, it may be held for various periods of time in different components of the cycle. A component of a biogeochemical cycle that holds an element or water for a short period of time is called an exchange pool. The atmosphere is an exchange pool for water. It usually holds water (in the form of water vapor) for just a week or two. A component of a biogeochemical cycle that holds an element or water for a long period of time is called a reservoir. The deep ocean, for example, is a reservoir for water. It may hold water for thousands of years. The rest of this concept takes a closer look at four particular biogeochemical cycles: the water, carbon, nitrogen, and phosphorus cycle. Water Cycle Water is essential to all living things on Earth, because virtually all biochemical reactions take place in water. Water can dissolve almost anything, so it also provides an efficient way to transfer substances between and within cells. The water cycle (also known as the hydrological cycle) describes the continuous movement of water on, above, and below Earth’s surface. As it cycles, water moves from one exchange pool or reservoir to another. In different parts of the cycle, water exists as a liquid (water), solid (ice), or gas (water vapor). Therefore, the water cycle includes several physical processes by which water changes state. Introduction to Environmental Chemistry Page 7 Like other biogeochemical cycles, the water cycle has no beginning or end. It just keeps repeating. Note that the term "evapotranspiration" in this diagram refers to the evaporation of water from Earth's land and water, along with the transpiration of water from the leaves of plants. Evaporation, Sublimation, and Transpiration As water passes through the water cycle, it can change to the gaseous state via three different processes: evaporation, sublimation, and transpiration. These three processes move water into the atmosphere. Evaporation occurs when water on Earth’s surface changes to water vapor. When the sun heats water, it gives water molecules enough energy to escape into the atmosphere. Water evaporates from soil on Earth’s surface, as well as from bodies of water. When salty ocean water evaporates, it leaves the salt behind. This process changes salt water to fresh water, which can then replenish the land. Sublimation occurs when ice and snow change directly to water vapor without first melting to form liquid water. Sublimation also occurs because of heat from the sun. Introduction to Environmental Chemistry Page 8 Transpiration occurs when plants release water vapor through leaf pores called stomata (shown in the figure below). Plants take up more water through their roots than they need for photosynthesis and other processes. Much of this excess water is given off via transpiration. In the process of transpiration, the many tiny stomata in plant leaves release water vapor into the atmosphere Condensation and Precipitation The relatively low density of molecules of water vapor causes water vapor to rise up into the atmosphere. As the water vapor rises higher above Earth’s surface, it cools and condenses on tiny dust particles in the air. Condensation is the process in which water vapor changes to liquid water, forming water droplets. If enough water droplets are present, they may form a visible cloud. If the droplets become large enough, they fall to Earth because of gravity as precipitation, such as rain, snow, sleet, or hail. Most precipitation falls into the ocean, because ocean water covers much of Earth’s surface. Eventually, this water evaporates again and repeats the cycle. Some frozen precipitation becomes part of ice caps and glaciers. These masses of ice can store frozen water for hundreds or even thousands of years. Introduction to Environmental Chemistry Page 9 Groundwater and Runoff Precipitation that falls on land may flow over the surface of the ground. This water is called runoff, and it may eventually flow into a body of water. As water flows over land, it dissolves minerals and carries them across Earth’s surface. Flowing water also causes erosion and deposition of eroded sediments. These processes reshape Earth’s geological features. Some of the precipitation that falls on land may soak into the ground and become groundwater. Groundwater may seep out of the ground at a spring or into a body of water, such as a lake or the ocean. Some groundwater may be taken up by plant roots. Some may flow deeper underground to an aquifer. As illustrated in the figure below, an aquifer is an underground layer of water-permeable porous rock that is saturated with ground water. It sits atop an impermeable rock layer that prevents the water from seeping further underground. This allows the aquifer to store water, sometimes for thousands of years. Aquifers provide a reservoir of fresh water that can be used by people if it is tapped by wells. Carbon Cycle Carbon is the basis of life on Earth. Chains of carbon bonded together form the backbone of many biochemical molecules. Carbon is also an important component of rocks and minerals, and carbon exists in the atmosphere in compounds such as carbon dioxide. The carbon cycle is the biogeochemical cycle in which carbon moves through the biotic and abiotic components of ecosystems. The carbon cycle is represented by the diagram in the figure below. Introduction to Environmental Chemistry Page 10 In the carbon cycle, carbon moves from one carbon store to another Carbon cycles quickly between organisms and the atmosphere. Cellular respiration by living things releases carbon into the atmosphere as carbon dioxide. Photosynthesis by producers (such as plants) removes carbon dioxide from the atmosphere and uses it to make organic carbon compounds. Carbon in organic compounds moves through ecosystem communities from producers to consumers, as modeled by food chains and food webs that show feeding relationships. Carbon is also released back into the environment when organisms decompose. Several human actions release huge amounts of additional carbon into the atmosphere. The most significant of these actions is the burning of fossil fuels. Large amounts of carbon in the gas methane are also released into the atmosphere from the decomposition of livestock manure and the wastes in landfills. Some natural events can also quickly add carbon to the atmosphere. Wildfires produce carbon dioxide as a product of combustion, and volcanic eruptions release carbon dioxide from molten rock (magma). Large volcanic eruptions (like the one in the photo below) can release enormous amounts of carbon dioxide in a short period of time. Carbon generally cycles more slowly through other processes. Running water, for example, slowly dissolves carbon in rocks, and most of this carbon ends up in the ocean. The top layer of ocean Introduction to Environmental Chemistry Page 11 water dissolves some carbon dioxide out of the atmosphere, and carbon also enters ocean water from the decomposition of aquatic organisms. Carbon from these sources may settle to the bottom of the ocean as sediment. Over millions of years, this carbon may form fossil fuels or carboncontaining rocks. Carbon can remain in these reservoirs for millions of years. In the Earth’s atmosphere, carbon exists as carbon dioxide in 0.03 percent level by volume. This level is currently being shifted towards excess due to imbalance of anthropogenic activities such as excessive combustion of fossil fuels and deforestation. Some of the ways by which CO2 is released into the atmosphere are: A) Respiration of plants and animals: This is an exothermic reaction involving the breaking down of organic molecules, e.g. glucose, into carbon dioxide and water. C6 H12 O6 + 6O2 6CO2 + 6H2O + Energy B) Decay of plants and animals: Fungi and bacteria breakdown the carbon compounds i.e. carbohydrates, proteins and lipids in dead plants and animals, and convert the carbon to carbon dioxide in the presence of oxygen or carbon dioxide and methane (CH4) in the absence of oxygen e.g. C) Fermentation of carbohydrates: The enzymatic decomposition of carbohydrates produces CO2 as a by-product. D) Burning of fossil and agro fuels: Combustion of fossil fuels like petroleum products, coal, and natural gas and agro fuels releases CO2 (and water vapour) into the atmosphere. E) Thermal decomposition of carbonate rocks or limestone: When limestone soils are heated up or during the production of cement, CO2 is released into the atmosphere. Introduction to Environmental Chemistry Page 12 F) Warming of surface waters: This leads to the releasing of dissolved CO2 back into the atmosphere. G) Volcanic eruptions: During volcanic eruptions, the volcanic gases released into the atmosphere include water vapour, CO2 and SO2. The Ways by which CO2 is removed from the Atmosphere are: A) Photosynthesis: Primarily, photoautotrophs (plants and algae) use light energy to convert CO2 and water to organic molecules like glucose and other carbohydrates. To a less extent, chemoautotrophs (bacteria and archaea) convert CO2 and water to organic matter using energy derived from the oxidation of molecules of their substrates. B) Formation of carbonic acid: Carbon dioxide dissolves in rain water and droplets pass through the atmosphere. Also, at the surface of the oceans towards the poles where sea water becomes cooler, carbon dioxide dissolves in water to from carbonic acid. Carbonic acid reacts with weathered silicate rocks to produce bicarbonate ions which are used to make marine carbonates. C) Conversion of carbon to tissues and shells: Organisms in upper ocean areas of high biological productivity convert reduced carbon to tissues or shells. D) In the oceans, the major carbon reservoir is the inorganic carbon: When CO2 dissolves in water, a hydrated CO2 molecule is produced which then forms an equilibrium mixture containing bicarbonate (𝐻𝐶𝑂3− ) and carbonate (𝐶𝑂32− ) ions. At pH’s lower than those found in sea water, carbonic acid (H2CO3) will also be present. This can be summarized as: The overall reaction that takes place when CO2 dissolves in sea water can be summarized as: Introduction to Environmental Chemistry Page 13 Nitrogen Cycle Nitrogen makes up 78 percent of Earth’s atmosphere. It is also an important element in living things. Nitrogen is needed for proteins, nucleic acids, and many other organic molecules, including chlorophyll, without which plants and other photoautotrophs could not carry out photosynthesis. The nitrogen cycle is the biogeochemical cycle that recycles nitrogen through the biotic and abiotic components of ecosystems. The figure below shows how nitrogen cycles through a terrestrial ecosystem. Nitrogen passes through aquatic ecosystems in a similar cycle. This figure shows how nitrogen cycles between the atmosphere, soil, and living things in a terrestrial ecosystem. The only part of the cycle that does not occur naturally is the addition of nitrogen compounds to the soil in fertilizer. Plants cannot use nitrogen gas in the air to make organic compounds for themselves and the organisms that consume them, but they can use nitrogen in the form of compounds (such as Introduction to Environmental Chemistry Page 14 nitrates), which they can absorb through their roots. The process of changing nitrogen gas to nitrates is called nitrogen fixation. It is carried out by bacteria, called nitrogen-fixing bacteria that live in soil or on the roots of legumes such as peas. Nitrogen fixation is the primary source of nitrogen used by plants in most ecosystems. When plants and other organisms die or release wastes, decomposers break down their organic compounds. In the process, they release nitrogen in the form of ammonium ions into the soil. The ammonium ions can be absorbed by plant roots. The ions can also be changed to nitrates by soil bacteria called nitrifying bacteria. Not all of the nitrates produced by nitrogen-fixing and nitrifying bacteria are used by plants. Some of the nitrates are changed back to nitrogen gas by soil bacteria called denitrifying bacteria. This nitrogen returns to the atmosphere, thus completing the cycle. The basic processes of the nitrogen cycle are described as follows: 1. Nitrogen Fixation: This is the process by which the atmospheric nitrogen is converted into a form that is readily available to plants and subsequently to animals and humans. There are four ways of converting atmospheric nitrogen (N2) into more biochemically available forms. A) Biological Fixation: Symbiotic bacteria, e.g. Rhizobium, associated with the root nodules of leguminous plants and some free-living bacteria, e.g. Azotobacter, are able to covert (fix) free nitrogen to organic nitrogen. B) Industrial Fixation: In the industrial Haber-Bosch process, atmospheric nitrogen and hydrogen (obtained from natural gas or petroleum) are combined to form ammonia, NH3. The ammonia produced can be used to make fertilizers and explosives. Introduction to Environmental Chemistry Page 15 C) Combustion of fossil fuels: The exhaust fumes from internal combustion engines are made up of volatile matters including oxides of nitrogen. D) Electrical storms (lightning) and photolysis: During electrical storms, nitrogen is oxidized to NO, which is oxidized by ozone in the atmosphere to form NO2. NO2 in turn is reduced back to NO by photolysis. Once nitrogen has been fixed it can be assimilated by organisms or oxidized to 𝑁𝑂2− /𝑁𝑂3− , nitrification. + 2. Assimilation: Plants can absorb NO− 3 or NH4 ions from the soil (Nitrogen uptake) through their roots. Absorbed nitrate is first reduced to nitrite ions and then ammonium ions for subsequent incorporation into amino acids, nucleic acids and chlorophyll. In leguminous plants with root nodules, nitrogen in the form of ammonium ions can readily be assimilated. Animals and human beings are incapable of utilizing nitrogen from the atmosphere or inorganic compounds hence; they depend on plants or other animals (except ruminants) that feed on plants, for their protein. 3. Ammonification: Involves breakdown of organic nitrogen compounds into NH3 or 𝑁𝐻4+ . At death, the proteins stored in the body of plants and animals become waste materials. Urine contains the nitrogen resulting from the metabolic breakdown of proteins in form of urea, (NH2)2CO. Urea is rapidly hydrolyzed by the enzyme to ammonium carbonate, (NH4)2CO3. 4. Nitrification: Describes the oxidation of NH3 or NH4+ to NO2− or NO− 3 by organisms. The excess ammonia released by bacterial action on urea and proteins that are not used by plants is oxidized by the autotrophic nitrifying bacteria-Nitrosomonas and Nitrobacter. Introduction to Environmental Chemistry Page 16 Under aerobic conditions, Nitrosomonas convert ammonia to nitrite while nitrite is further oxidized to nitrate by Nitrobacter. 5. Denitrification: Nitrate and nitrite are reduced under anaerobic conditions by pseudomonas and clostridium bacteria. Nitrate is reduced to nitrite while nitrite is reduced to ammonia. Most of the nitrate is later reduced to nitrogen thus, completing the nitrogen cycle. Denitrification constitutes a serious loss of fertilizing matter in soil when anaerobic conditions develop. Phosphorus Cycle Phosphorus is an essential nutrient for plants, animals, and other organisms. Organic molecules that contain phosphorus include DNA, RNA, ATP, and the phospholipids that make up cell membranes. Phosphorus is also found in human bones and teeth, and it helps the human body maintain acidbase homeostasis. The phosphorus cycle, do not have a gaseous component and is an endogenic cycles which predominantly involves subsurface rocks of various kind which makes it different from other cycles. The phosphorus cycle is the biogeochemical cycle in which phosphorus moves through rocks, water, and living things. Unlike many other biogeochemical cycles, the atmosphere does not play a significant role in the cycling of phosphorus, because phosphorus and phosphorus-based compounds are not gases at the typical ranges of temperature and pressure found on Earth. The way phosphorus cycles through terrestrial and aquatic ecosystems is illustrated in the following figure. Phosphorus cycles quickly through living things, but very slowly through the abiotic components of ecosystems, making the overall phosphorus cycle one of the slowest biogeochemical cycles. Introduction to Environmental Chemistry Page 17 Besides processes in living things, the phosphorus cycle includes geological processes of weathering, erosion, and sedimentation On land, most phosphorus is found in rocks. Weathering of rocks releases phosphorus in a soluble form that can be absorbed from soil by plant roots. Plants then use the phosphorus to make organic compounds, which can be passed on to consumers through feeding relationships. After organisms die, the phosphorus they contain is returned to soil by decomposers. Plants must quickly take up this phosphorus before it is transformed into insoluble compounds by reacting with other minerals in soil. Runoff may also carry away some of the phosphorus in soil before plants can use it, and this phosphorus may eventually end up in the ocean. Over time (generally thousands of years), soil tends to become deficient in phosphorus. That’s because the addition of phosphorus to soils by weathering generally cannot keep pace with its loss in runoff and chemical reactions with other soil minerals. A low concentration of phosphorus in soil, in turn, reduces plant growth. Fortunately for many plants and the other organisms that depend Introduction to Environmental Chemistry Page 18 on them, certain fungi that grow on plant roots (see the photo below) secrete acid that releases phosphorus from insoluble compounds in soil, making the phosphorus available to plant roots. On farmland where phosphates are applied as fertilizers, plants do not absorb all phosphate. The phosphates end up in water and stream towards lakes and reservoirs, where they cause a phenomenon called eutrophication. Eutrophication means that the water is so rich in nutrients that it causes certain water plants, such as green algae, to grow extensively. Phosphorus that plants can use may also be added to soil in fertilizers. To sum up, Water and the chemical elements that organisms need continuously cycle through ecosystems. Cycles of matter are called biogeochemical cycles, because they include both biotic and abiotic components and processes. Components that hold matter for short periods of time are called exchange pools, and components that hold matter for long periods of time are called reservoirs. The water cycle involves water changing state as it moves from one exchange pool or reservoir to another. Water changes to water vapor and enters the atmosphere through evaporation, sublimation, and transpiration. Water vapor in the atmosphere changes to liquid water by condensation, which may form clouds and fall back to Earth as precipitation. Water that evaporates from the ocean renews Earth’s source of fresh water, because the salt is left behind. Liquid precipitation may form runoff that eventually flows into a body of water, or it may soak into the ground and become groundwater. Some groundwater is stored in aquifers, which are layers of porous rock atop impermeable rock layers and which can be tapped with wells for human use. Frozen precipitation may become part of ice caps or glaciers that store fresh water for long periods of time. Carbon cycles quickly between organisms and the environment through cellular respiration and photosynthesis. Carbon in organic compounds moves through food chains and webs, and some is released back to the environment by decomposers. Human actions (such as burning fossil fuels) release huge amounts of additional carbon dioxide into the atmosphere. Introduction to Environmental Chemistry Page 19 Most natural processes cycle carbon more slowly. Running water slowly dissolves carbon in rocks and carries it to the ocean, and the top layer of ocean water dissolves carbon dioxide out of the atmosphere. Carbon in ocean water may gradually settle to the bottom, and some of this carbon may eventually be changed to fossil fuels or sedimentary rocks that can store carbon for millions of years. Nitrogen gas in the atmosphere cannot be used by plants, but nitrogen-fixing bacteria in soil or on plant roots change nitrogen gas to nitrates, which plant roots can absorb. Decomposition of organic matter releases nitrogen as ammonium ions that plants can also absorb, or that nitrifying bacteria in soil can change to nitrates for use by plants. Denitrifying bacteria release nitrogen gas from unused nitrates, and this nitrogen enters the atmosphere and completes the cycle. Phosphorus slowly enters soil by the weathering of rocks. Plants absorb phosphorus in soil and pass it on to consumers, and decomposers eventually return it to soil. Some phosphorus in soil is carried away by runoff, and some binds with other minerals to form insoluble compounds that plant roots cannot absorb. Soil generally becomes deficient in soluble phosphorus over thousands of years. Certain fungi that grow on plant roots release phosphorus from insoluble compounds so plant roots can absorb it. Introduction to Environmental Chemistry Page 20