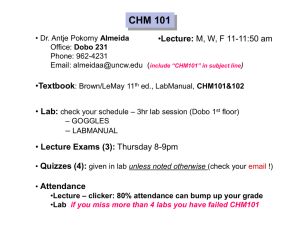
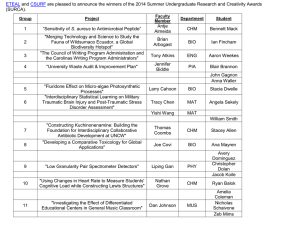
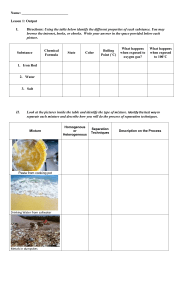
Grade 09 CHM 41 Chapter 1 - More Practice Sheet Topic 1 Practice Questions QP Subtopic 1.1 Elements, Mixtures & Compounds 1. Which of the following is/are example(s) of matter? A. Fog B. Lamp C. Helium gas D. All of the above Consider the following particulate diagrams to answer questions 5 and 6. 5. Which of the above figures is/are considered a mixture? 2. All of the following are polyatomic elements except ____________. A. Zn B. S8 C. O3 D. P4 A. K only B. M only C. L and M only D. K, L and M 6. Which of the above diagrams is a pure substance? 3. Which of the following is a polyatomic element? A. L only A. S8 B. M only B. I2 C. K and L only C. Ne D. K, L and M D. Cu ____________________________________________________ 4. Which of the following is a diatomic element? 7. Tap water is an example of a(n) ______. A. element A. S8 B. pure substance B. P4 C. homogenous mixture C. Xe D. heterogeneous mixture D. I2 ___________________________________________________ Topic 1 Practice Questions QP 1 Grade 09 CHM 41 8. Which of the following is a heterogeneous mixture? A. Air B. Water C. Alloy D. Sea water Use the following diagram to answer questions 11 and 12. ______________________________________________________ 11. Which of the above diagrams represents a compound? Use the following diagram to answer questions 9 and 10. 9. Which of the following best describes Figure A? A. W B. X C. Y D. Z 12. Which of the above diagrams represents a mixture of an element and a compound? A. Element A. W B. Mixture B. X C. Solution C. Y D. Compound D. Z 13. Matter is best described as anything that has _________________ only. 10. Which of the following statements is true? A. Figure B is composed of two different compounds B. Figure C is composed of two different compounds C. Figure C is composed of three different types of elements D. All of the above A. mass B. mass and velocity C. mass and takes up space D. mass, density and exerts pressure ______________________________________________________ Topic 1 Practice Questions QP 2 Grade 09 CHM 41 14. Which of the following is/are properties of a pure substance? I. Chemically inert II. Has a fixed composition III. Can be an element or a compound 18. Which of the following represents a mixture of three different elements? A. X only B. Y only A. I only C. Z only B. I and II only D. X and Y only C. II and III only D. I, II and III ________________________________________________ Use the following diagram to answer questions 19 and 20. Consider the following diagrams to answer questions 15 18. 19. Which of diagram represents(s) an element? 15. Which of the diagrams (X, Y and Z) represent(s) homogenous mixture? A. B. C. D. X only Y only X and Y only Y and Z only 16. Which of the diagrams (X, Y and Z) represent(s) pure element? A. B. W only Z only C. X and Z only D. X, Y and Z only 20. Which of diagram represents(s) a compound? Z only A. B. W only Z only C. X and Y only C. X and Z only D. Y and Z only D. X, Y and Z only A. X only B. ______________________________________________________ 17. Which of the following could be Z? A. Water B. Salt and water C. Hydrogen gas D. Phosphorous Topic 1 Practice Questions QP 3 Grade 09 CHM 41 21. Which of the following is/are example(s) of an element? I. S8 II. Fe III. Cl2 A. B. C. D. 24. Which of the above particulate diagrams represents carbon dioxide, CO2, molecules? I only II only I and II only I, II and III A. X only B. Y only C. Z only D. X and Y only 25. Which diagram(s) represent(s) a pure substance? ______________________________________________________ Consider the following particulate diagrams (X, Y and Z) to answer questions 22 25. A. X only B. Y only C. Y and Z only D. X, Y and Z ______________________________________________________ 26. All of the following substances are elements except _________. 22. Which diagram represents helium, He, atoms? A. X only B. Y only C. Z only D. X and Y only mixture B. element C. compound D. pure substance Topic 1 Practice Questions QP water B. oxygen C. chlorine D. nitrogen 27. Which of the following is not a compound? 23. Diagram X represents a(n) _______________. A. A. A. O2 B. CO C. CO2 D. NO 28. Bronze is considered as a __________. 4 A. pure element B. pure compound C. homogenous mixture D. heterogeneous mixture Grade 09 CHM 41 29. Air is a ________ mixture in which different substances are _______ mixed. A. heterogeneous evenly B. heterogeneous not evenly C. homogeneous not evenly D. homogeneous evenly Use the following particulate diagram to answer questions 33 and 34. 33. The particulate diagram that represents a heterogeneous mixture is ________ because the components are _______ mixed. 30. Sea water is a _______ mixture in which different substances are ______ mixed. A. heterogeneous evenly B. heterogeneous not evenly C. homogeneous evenly D. homogeneous not evenly I only B. II only C. I and II only D. I, II and III N not evenly B. Q evenly C. W evenly D. Q not evenly 34. The particulate diagram that represents a homogeneous mixture is _______ because the components are _________ mixed. A. N not evenly 31. Which of the following mixtures is/are heterogeneous? I. Sand and water II. Orange juice with pulp III. Sodium chloride dissolved in water A. A. B. Q evenly C. W evenly D. Q not evenly ___________________________________________________ 32. Which of the following is/are correct safety precaution(s) when heating flammable liquids? I. Use a hot plate II. Wear safety goggles III. Always tie any long hair A. B. I only II only C. I and III only D. I, II and III Topic 1 Practice Questions QP 5 Grade 09 CHM 41 35. Classify each of the following diagrams as element, mixture or compound. If any is a mixture specify its type. ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ 36. Classify the following as pure substance, homogeneous mixture or heterogeneous mixtures. a) Air __________________________________________ b) Carbon dioxide __________________________________________ c) Distilled water __________________________________________ d) Sand and water __________________________________________ e) Vinegar and water __________________________________________ f) Table salt __________________________________________ g) Blood __________________________________________ h) Brass __________________________________________ Topic 1 Practice Questions QP 6 Grade 09 CHM 41 37. Consider the following particulate diagrams to answer questions a c. a) Write the letter (P, R, S, T or U) that matches each of the following statements. (Each letter may be used once, more than once or not at all) A mixture of an element and a compound _________________ A mixture of more than one element _________________ A heterogeneous mixture _________________ A pure element _________________ b) Which letter (P, R, S, T or U) represents a homogeneous mixture? Explain your answer. c) Which letter (P, R, S, T or U) represents a pure compound? Justify your answer. 38. Use the particulate diagrams below to identify the letter (Q, R, S, T or U) that best fits each of the following statement. (Each letter may be list once, more than once or not at all) a) These are elements ____________________ b) This is a solution ____________________ c) This is a compound ____________________ Topic 1 Practice Questions QP 7 Grade 09 CHM 41 39. Use the following figure to identify the letter (A I) that fits each of the following. (Each letter may be used once, more than once or not at all) Element ________________ Compound ________________ Mixture ________________ Mixture of a monoatomic element and a diatomic element ________________ This could be a mixture of He and H2 ________________ This could be H2O ________________ This could be N2 ________________ This could be a mixture of Na and HCl ________________ Topic 1 Practice Questions QP 8 Grade 09 CHM 41 40. Consider the three different substances given below to answer questions a c. (Each substance may be used once, more than once or not at all) a) Which of the above is/are pure substance(s)? b) Which of the above is an element? c) A student predicted that sea water is a heterogeneous mixture. Is the student correct? Justify your answer. 41. Write the name of the laboratory equipment needed for each of the following. Used to provide heat _________________ Used to hold an evaporating dish _________________ Important eye equipment for lab work _________________ Used to transfer small amounts of solid _________________ Used to measure volume of liquid accurately _________________ You must always wear these to protect your hands _________________ Used to hold solids or liquids and carry out reactions _________________ Topic 1 Practice Questions QP 9 Grade 09 CHM 41 42. In the table below, write the name of the laboratory equipment that is needed for each of the following uses. Name of Laboratory Equipment Use Protects the eyes from flying objects or chemical splashes A wide-mouthed container used to transport, heat or store substances P ec he cie i a d he cie i cl he f hazardous or hot chemicals Used to dispense a very small amount of a liquid Used to pour liquids into containers with small openings or to hold filter paper 43. A student found the following labels on a bottle in the Chemistry lab. Write the name of each hazard sign. ______________________ Topic 1 Practice Questions QP ______________________ 10 ______________________ Grade 09 CHM 41 44. On a school day, a Grade 09 student had a chemistry class in his chemistry lab. He shared with his parents his experience during that day. Unfortunately, there were some mistakes in his actions during the chemistry lab. Read the passage below and identify the mistakes of the students. Propose a correct action for each mistake the student have done. Today I had a chemistry class. The teacher took us to the chemistry lab. I forgot my lab coat that day. When we went to the lab, I put on my gloves and I wore my eye goggles although I was wearing my lenses. The teacher asked us to carry out an experiment were we have to heat a liquid over the Bunsen burner. My colleague and I collected the apparatus needed: Test tube, tongs, and Bunsen burner. My colleague took a test tube and completely filled it with the liquid to be heated. He heated it vertically over the Bunsen burner without moving it. After a while the liquid started splashing out of the test tube and some drops came on my colleague hand. He quickly wiped it with a tissue. The teacher then came to us and told us that we have done some mistakes during the lab that she needs to discuss this with us later. Incorrect Action 1: _______________________________________________________________________________________. Correction: ________________________________________________________________________________________________. Incorrect Action 2: _______________________________________________________________________________________. Correction: ________________________________________________________________________________________________. Incorrect Action 3: _______________________________________________________________________________________. Correction: ________________________________________________________________________________________________. Incorrect Action 4: _______________________________________________________________________________________. Correction: ________________________________________________________________________________________________. Incorrect Action 5: _______________________________________________________________________________________. Correction: ________________________________________________________________________________________________. Topic 1 Practice Questions QP 11 Grade 09 CHM 41 45. Identify five safety hazards in the figure below from a lab. Topic 1 Practice Questions QP 12 Grade 09 CHM 41 46. Name the lab apparatus or equipment that fits each of the following functions. Suitable Lab Apparatus or Equipment Function o Used to hold crucibles or hot objects o Protects the eyes from flying objects or chemical splashes o Used to heat objects o Used to pour liquids into containers with small openings or to hold filter paper o A small glass container used to view chemical reactions or to heat small amounts of a substance o A wide-mouthed container used to transport, heat or store substances; not reliable for accurate volume measurement o A narrow-mouthed container used to transport, heat or store substances, often used when a stopper is required 47. Name the apparatus that can be used to perform each of the following task. Suitable Lab Apparatus Function o Holding 120. mL of water o Measuring 27 mL of a liquid o Measuring exactly 34 mL of an acid o Measuring out 130 g of sodium chloride o Mixing small amount of solids together o Holding many test tubes filled with chemicals Topic 1 Practice Questions QP 13 Grade 09 CHM 41 48. Decide whether each of the following statements is true (T) or false (F). o While people who participate in extreme sports must think about safety all the time, scientists never need to consider it. __________ o Long, loose hair and lots of dangling jewelry is appropriate chemistry lab attire __________ o A messy workspace is safer, and may give you inspiration to do good science work __________ o It is very important to always follow directions. __________ o Glassware is fragile, so you should be sure to place it away from the edge of the table to prevent it from being knocked over. __________ o Gloves help protect your hands from harmful substances. __________ o It is best to assume that glassware is never hot, because it does not appear hot. __________ o Science labs are rarely equipped with fi re extinguishers, because fires do not happen in science labs. __________ Topic 1 Practice Questions QP 14 Grade 09 CHM 41 49. During the practical lesson in a Chemistry laboratory, the students must wear specific safety clothes and they use different laboratory glassware or equipment. Complete the following table by writing the missing name or its use. Laboratory Equipment Name Use Used to hold solids or liquids and carry out reactions Important eye equipment for lab work Pipette Used to transfer small amounts of solid Used to provide heat Topic 1 Practice Questions QP 15 Grade 09 CHM 41 Subtopic 1.2 Properties of Matter 1. Which of the following is/are physical property? I. Conductivity II. Flammability III. Boiling point A. I only B. II only C. III only D. I and III only 4. Consider the following reaction: W+Z WZ. If 35.0 g of W combines completely with Z to form 60.0 g of WZ, what is the mass of Z needed? Silver metal reacts with acids B. Sugar turning into brown solid when heated C. Aluminum can be hammered into thin sheets D. Sodium reacts vigorously when dropped in water 25.0 g B. 35.0 g C. 60.0 g D. 95.0 g 5. The figures below represent the particulate diagrams of two different changes X and Y. Which of the following correctly identifies the changes X and Y? 2. Which of the following is not a chemical property? A. A. 3. Hydrogen combines with nitrogen to form ammonia. Which of the following correctly lists the reactants and products of this reaction? A. Reactant(s) Product(s) Hydrogen Nitrogen and ammonia Nitrogen and Hydrogen Hydrogen B. Ammonia C. Nitrogen and ammonia D. Nitrogen and hydrogen Topic 1 Practice Questions QP Ammonia 16 X Y A. Physical change Chemical change B. Chemical change Physical change C. Physical change Physical change D. Chemical change Chemical change Grade 09 CHM 41 13. Which of the following indicates that a chemical reaction occurred? I. The temperature of mixture increased by 21℃ II. The color of a solution changed from orange to green III. A solid substance is formed after mixing two solutions A. I only B. I and II only C. II and III only D. I, II and III 16. Which of the following is/are considered physical change(s)? I. Rusting of iron II. Evaporation of water III. Crushing salt crystals I only I and II only C. I and III only D. I, II and III Cutting a sheet of paper C. Crushing an aluminum can D. Reacting carbon with oxygen to form carbon dioxide B. II only C. III only D. II and III only A. I only B. II only C. I and II only D. I, II and III 18. All of the properties below are physical except _______________. 15. Which of the following is not a physical change? A. Breaking a conical flask B. I only 17. Which of the following is/are considered chemical change(s)? I. Rusting of iron II. Burning a candle III. Crushing salt crystals 14. A chemical reaction is a process that involves ___. I. formation of new substances II. rearranging the atoms in the reactants III. changing the type of atoms in the reactants A. B. A. A. odor B. color C. density D. flammability 19. Which of the following is a physical property? A. B. C. D. Topic 1 Practice Questions QP 18 Color Combustion Flammability Reactivity with water Grade 09 CHM 41 25. A student listed different properties of a substance in his lab report. Classify each of the following as physical or chemical properties. Is a gas Flammable Is colorless Highly reactive Has low boiling point Complete the diagram below by adding the property in the correct place. Physical Property Chemical Property 26. A substance is formed from its component elements according to the following reaction: X+Y XY. Answer questions a c. a) If 45.0 g of substance X reacts with substance Y to form 74.0 g of XY. What is the mass of B? (You must show all your work to earn the full mark) b) List and define the law that governs the above chemical reaction. c) Identify the reactant(s) and product(s). Reactant(s) ____________________________ Product(s) ____________________________ Topic 1 Practice Questions QP 20 Grade 09 CHM 41 27. Classify each of the following statements as a chemical property or a chemical change. Gold is an unreactive metal ______________________________ Gasoline is a highly flammable liquid ______________________________ Zinc reacts with an acid to produce hydrogen gas ______________________________ Magnesium burns in oxygen to produce a bright white flame ______________________________ Sodium reacts with water to produce bubbles of hydrogen gas ______________________________ 28. Classify each of the following changes as physical or chemical change. For each chemical change list one indication or evidence of the chemical change. a) 10.0 g of salt dissolves readily in 100 ml water. b) Aluminum metal can be hammered into thin sheets. c) Charcoal burns in a grill. 29. Classify each of the following changes as a chemical change (C) or a physical change (P). a) Evaporation of alcohol ______________________________ b) Crushing crystals of a solid ______________________________ c) Burning gasoline in car engine ______________________________ Topic 1 Practice Questions QP 21 Grade 09 CHM 41 35. Complete the diagram below by writing the letter of statements (Q, L, M or N) in the correct circle. Q Iron melts at 538℃ L Grinding a piece of solid speeds up the reaction M Magnesium ribbon can be cut down into smaller pieces N Potassium reacts with water and produces hydrogen gas Topic 1 Practice Questions QP 25 Grade 09 CHM 41 36. Read the following passage and use your knowledge about physical and chemical properties and changes as well as intensive and extensive properties to answer questions a and b. Sodium is a chemical element with the chemical symbol Na and atomic number 11. Compared to other metals, sodium is soft and have a silvery white color, melts at 97.8 C and boils at 882.9 C and has a density of 0.968 g/cm3. Sodium is a highly reactive metal. It reacts violently with water where hydrogen gas is produced. Sodium hardly reacts with carbon, but it does react with halogens to form sodium salts. Sodium is the sixth most abundant element in he Ear h s cr s Sodi m is af er chloride he second most abundant element dissolved in seawater. a) b) i. List two physical properties of sodium. ii. List two chemical properties of sodium. i. Write one intensive property of sodium. ii. List from the above paragraph one indicator of a chemical change. Topic 1 Practice Questions QP 26 Grade 09 CHM 41 37. Read the following passage about sulfur then answer questions a c. Sulfur element is an odorless, tasteless, light yellow solid at room temperature. It melts at 115 C, boils at 444 C and has a density of 2.07g/cm3. Solid sulfur is combustible and molten sulfur is flammable. Also, sulfur is a reactive element that can combine with many other elements except gold and platinum. a) List two physical properties of sulfur. 1) _____________________________________________________________ 2) _____________________________________________________________ b) List two chemical properties of sulfur. 1) _____________________________________________________________ 2) _____________________________________________________________ c) When burning sulfur in the presence of oxygen, a gas will be produced. Is this change physical or chemical? Justify your answer. Topic 1 Practice Questions QP 27 Grade 09 CHM 41 Subtopic 1.3 States of Matter Consider the following particulate diagrams Q and P to answer questions 1 and 2. 1. Which of the following correctly states that state of P and Q? A. P is solid and Q is gas B. P is gas and Q is liquid C. P is liquid and Q is gas D. Both P and Q are gases I only B. II only C. I and II only D. II and III only Topic 1 Practice Questions QP A. I only B. II only C. III only D. I, II and III 4. Deposition is the change of state from _______ to ________. 2. Which of the following is true about P and Q? I. Particles in P are compressible II. Particles in P have regular arrangement III. Particles in Q have low density and can be compressed A. 3. Which of the following is/are true of gases? I. Indefinite shape II. Indefinite volume III. High compressibility A. solid liquid B. liquid gas C. gas liquid D. gas solid 5. Solid has a(n) __________________. A. indefinite shape B. indefinite volume C. high compressibility D. regular arrangement of particles 6. Which of the following best describes hydrogen gas? 28 A. Low density B. Has a definite volume C. Particles slide past one another D. Regular arrangement of particles Grade 09 CHM 41 13. Methane, CH4, has a melting point and boiling point below 0℃. Methane is expected to be a _______ at room temperature. A. gas B. solid C. liquid D. plasma 15. During the process of ____________, heat is ________ a sample of a solid to change it to a liquid. A. melting added to B. melting removed from C. freezing added to D. freezing removed from 14. During the process of ____________, heat is ________ a sample of solid to change it to a gas. A. sublimation added to B. sublimation removed from C. deposition D. evaporation removed from added to 16. Use the following table to compare between the three states of matter in terms of shape, volume, and compressibility. State of matter Solid Liquid Gas Shape Volume Compressibility Topic 1 Practice Questions QP 30 Grade 09 CHM 41 17. Consider the following particulate diagram to answer questions a and b. a) Which letter represents the change that occurs by: Addition of heat ________________ Removal of heat ________________ b) List the letter that best fits each of the following statements. The state in which particles are highly compressible ________________ The state in which particles can slide past one another ________________ The state in which particles are closely packed together ________________ Evaporation ________________ Sublimation ________________ Deposition ________________ 18. Identify the state of matter: solid, liquid or gas, that best fits each of the following statements. a) Has an indefinite shape and definite volume ______________ b) Its particles vibrate in their fixed positions c) Its particles have irregular arrangement and move randomly ______________ Topic 1 Practice Questions QP 31 ______________ Grade 09 CHM 41 21. For each of the following changes of state, identify the initial and final state of matter and whether heat is added or removed. Change of state Initial State of Matter Final State of Matter Heat Added or Removed a) Melting b) Deposition c) Condensation 22. A student was asked to write in his Chemistry copybook about the three states of matter. Read the following paragraph and identify TWO errors in the paragraph below and write the correct statements. Matter has only two main states: solid and liquid. A solid has a definite shape but an indefinite volume. A liquid is highly incompressible and has regular arrangement of particles. a) Error 1: ___________________________________________________________________________________________________ Correct statement 1: _____________________________________________________________________________________ b) Error 2: _____________________________________________________________________________________________ Correct statement 2: ____________________________________________________________________________________ Topic 1 Practice Questions QP 33 Grade 09 CHM 41 39. Use the diagram below that represents three states of matter to identify the state of matter (X, Y or Z) that best fits each of the following statements. (Each letter may be used once, more than once, or not at all) a) The state of matter with a definite shape ______________ b) The state of matter that has the highest density ______________ c) The state of matter that is highly compressible ______________ d) Particles in this state of matter slide past each other ______________ Topic 1 Practice Questions QP 36 Grade 09 CHM 41 Subtopic 1.4 Separation techniques 1. Which of the following separation techniques can be used to separate an insoluble solid from a liquid? I. Filtration II. Fractional distillation III. Using separating funnel A. I only B. II only C. III only 5. The diagram below represents ______________, where oil is __________ than water that is why it can be separated from a mixture of oil and water at the end. D. I and II only _____________________________________________________ 2. Consider the following figure to answer questions 2 and 3. A. filtration denser B. separatory funnel lighter C. separatory funnel denser D. filtration lighter 6. A mixture of solid ammonium chloride and solid sodium chloride can be separated by ____________. 3. What is the name of the above separation technique? A. Filtration B. Distillation A. filtration C. Decantation B. decantation D. Centrifugation C. sublimation D. centrifugation 4. What are the physical states of substance R and T? R T A. Liquid Solid B. Solid Solid C. Solid Liquid D. Liquid Liquid ______________________________________________________ Topic 1 Practice Questions QP 37 Grade 09 CHM 41 7. The separation method in the diagram below can best be used to separate_________. A. a mixture of oil and water B. a mixture of water and ethanol a mixture of sulfur and iron copper sulfate crystals from copper sulfate solution C. D. Use the diagram below to answer questions 8 10. 9. This separation technique represented by the diagram above is called _______________. 8. Which of the following statements is/are true about the separation technique represented below? A. filtration B. centrifugation C. simple distillation D. fractional distillation 10. In K ______ takes place and in M _______ takes place. Liquid V is denser than liquid W It is used to separate two immiscible liquids It can be used to separate a mixture of kerosene oil and water I. II. III. A. I only B. II only C. I and II only D. II and III only Topic 1 Practice Questions QP A. evaporation deposition B. evaporation condensation C. condensation evaporation D. deposition condensation 11. The laboratory equipment L is called a ______________. A. beaker B. burette C. cylinder D. Florence flask ______________________________________________________ 38 Grade 09 CHM 41 12. The process of separation of insoluble materials from a liquid where normal filtration does not work well is called ________________. A. chromatography B. centrifugation C. distillation D. filtration Use the diagram below to answer questions 14 16. 13. The separation technique in the diagram can best be used to separate a mixture of ____________. A. sand and water B. alcohol and water C. camphor and water D. table salt and water 15. The separation technique in the diagram above can be used to separate _________. A. oil and water B. water and ethanol C. immiscible liquids D. different components in a liquid mixture 16. Dye ___________ is most soluble in the solvent and dye __________ is the least soluble in the solvent. A. 1 2 14. The separation technique in the diagram can best be used to separate a mixture of ____________. B. 1 3 C. 3 1 D. 3 2 17. Which dye(s) is/are present in the mixture? I. Dye 1 II. Dye 2 III. Dye 3 A. I only A. oil and water B. ethanol and water B. II only C. liquids of different colors C. III only D. I and III only ______________________________________________________ D. blood components ______________________________________________________ Topic 1 Practice Questions QP 39 Grade 09 CHM 41 18. Which of the following separation techniques can be used to separate two or more miscible liquids with close boiling points? A. Centrifugation B. Chromatography C. Simple distillation D. Fractional distillation _____________________________________________________________________________________________________________________ 19. Identify the separation technique that can best separate each of the following mixtures. a) Iron and sulfur _________________________ b) Oil and water _________________________ c) Iodine from a mixture of iodine and sand _________________________ Topic 1 Practice Questions QP 40 Grade 09 CHM 41 20. Salt is soluble in water, but sand is insoluble in water. This difference allows a mixture of salt and sand to be separated using this apparatus. Answer questions a – c. a) Use words from the box below to complete the sentences. (Each word may be used once, more than once or not at all) beaker funnel burette water glass rod thermometer Bunsen burner conical flask In Step 1, the mixture of salt and sand is placed in a ____________________________ containing _____________________________ and stirred with a ______________________. In Step 2, the mixture from Step 1 is poured through a _____________________________ into a _____________________________. In Step 3, the liquid is transferred to a basin to allow the. ____________________________ to be removed. b) i. What needs to be placed in A before the mixture from Step 1 is poured through it? ii. What is the solid removed in Step 2? c) List the names of two processes used in this separation. Topic 1 Practice Questions QP 41 Grade 09 CHM 41 21. Consider the following separation technique to answer questions a c. a) Name the method of separation represented by the figure above. b) Identify the change of state that happens at A and D. At A: _____________________________ At D: _____________________________ H: _____________________________ C: _____________________________ c) Label H and C. Topic 1 Practice Questions QP 42 Grade 09 CHM 41 22. Ahmed wanted to find out if some food colorings contained a banned food dye. He put a drop of each food coloring and the banned food dye onto some special paper. He hung the paper in a beaker of water as shown in the figure below. After 10 minutes, the banned food dye and some of the dyes from the food colorings had moved up he a e Ah ed e l a e h bel Topic 1 Practice Questions QP 43 Grade 09 CHM 41 Answer questions a f. a) Which method did Ahmed use to separate the dyes? Tick ONE correct box only. Filtration Distillation Evaporation Chromatography b) Identify the mobile phase and stationary phase. Mobile Phase: _________________________ Stationary Phase: _________________________ c) Ahmed used ink to draw the baseline. Is this step correct? Justify your answer. Ba ed Ah ed e l diffe e d e i e f he f d c l i g had ed he a e d) Which food coloring contained the banned dye? e) Which food coloring contained the most dyes? f) Which food coloring did not dissolve in water? Topic 1 Practice Questions QP 44 Grade 09 CHM 41 23. A student wanted to separate a mixture of salt and sand dissolved in water to obtain salt. He used two different separation techniques (P and Q) that are shown in the diagram below. Answer questions a c. a) Name the two separation techniques, P and Q. P: ____________________________ Q: _____________________________ b) Write the name of each of the following devices used in separation techniques P and Q. O: ____________________________ Y: _____________________________ c) The student added water to the sand and salt then stirred it very well. He decided to begin with separation technique P then Q in separating the salt solution and sand. Did the student take the right decision? Justify your answer. Topic 1 Practice Questions QP 45 Grade 09 CHM 41 24. Ahmed used the apparatus below to distil 100 cm3 of water-soluble ink. Answer questions a c. a) Which processes occur during distillation? Tick ONE correct box only. Condensation then evaporation Evaporation then condensation Melting then boiling Melting then evaporation b) Give the name of the colourless liquid that collects in the test-tube. c) What would the temperature reading be on the thermometer when the ink has been boiling for two minutes? _______°C Topic 1 Practice Questions QP 46 Grade 09 CHM 41 25. A student wanted to separate a mixture of water, sand and salt. He sketched the diagram below. Answer questions a and b. a) Fill in the table below. Letter Name of lab equipment Use To heat a liquid W Filter funnel P b) Describe the action the student needs to take if any of the following accidents occur. Accident Action taken The conical flask falls on the floor and breaks The solution is spilled on the floor The de c b k ca che fi e A drop of the salty water entered the eye Topic 1 Practice Questions QP 47 Grade 09 CHM 41 26. Identify the separation technique that best fits each of the following statements. a) Separates liquids with different boiling points _________________________ b) Separates a liquid and solid by boiling off the liquid _________________________ c) Separates liquids of different colors _________________________ d) Separates a solid that can dissolve in water from one that cannot _________________________ 27. Identify the separation technique from the box below that can be used to separate each of the following mixtures. (Each word may be used once, more than once or not at all) Filtration Simple Distillation Fractional Distillation Decantation Centrifugation Evaporation a) Separating a mixture of soil and water _______________________________ b) Separating salt from a mixture of salt, sugar and alcohol _______________________________ c) Separating a mixture of water and ethanol _______________________________ d) Separating pure water from a solution of sodium chloride _______________________________ Topic 1 Practice Questions QP 48 Grade 09 CHM 41 28. A student wanted to separate the different components in a liquid mixture. He carried out an experiment as shown in the diagram below. Answer questions a e. a) Name the separation technique represented by the diagram above. b) Which is the most soluble color in the solvent? c) Identify the stationary phase. d) Identify the mobile phase. e) Which color has the smallest Rf value? Topic 1 Practice Questions QP 49 Grade 09 CHM 41 29. A student wanted to separate a mixture of acetone and water. Use the diagram below to answer questions a e. a) Name the separation technique represented by the diagram. b) If the boiling point of acetone is lower than that of water, which component would be first collected in container M? c) The phase change that will take place in part L is _____________. d) Name the pieces of apparatus listed below. P: ____________________________ N: _____________________________ K: _____________________________ e) If the above apparatus is to be adjusted to separate water from table salt solution, what is needed to be done? Topic 1 Practice Questions QP 50 Grade 09 CHM 41 30. A student wanted to separate a mixture of chalk and water. He used the apparatus shown in the diagram below. Answer questions a d. a) Identify the filtrate. b) Identify the residue. c) Y is called _____________. d) The mixture of chalk and water is __________________. Topic 1 Practice Questions QP 51 Grade 09 CHM 41 Grade 09 – CHM 41 Topic 2 – Practice Questions – QP Subtopic 2.1 – Units of Measurement 1. Which is an S.I unit of time? 5. The average temperature of the human body is 36℃. This is equivalent to ______ Kelvin. A. Minute B. Second A. 273 C. Hour B. 298 D. Day C. 300 D. 309 2. The SI base unit of mass is __________. 6. The average room temperature is 25 C which is equivalent to ________ Kelvins. A. g B. kg C. cg A. 273 D. mg B. 298 C. 300 D. 309 3. The prefix pico is _____________ of the SI unit. A. 10 B. 10 C. 10 D. 10 12 7. What is the temperature of 5 K in Celsius? 3 4. The prefix Mega is __________ times larger than the unit it precedes. A. 10 B. 100 C. 1,000 D. 1,000,000 Topic 2 – Practice Questions QP A. 268℃ B. 273 ℃ C. 268℃ D. 278℃ 8. Which of the following units is the smallest? 1 A. Micrometer B. Kilometer C. Decimeter D. Millimeter Grade 09 – CHM 41 9. The S.I. unit of mass is ________. 12. The S.I. unit of length is ________. A. ounce A. meter B. pound B. decimeter C. newton C. centimeter D. kilogram D. millimeter 10. The prefix micro ______ the base unit ______ times. 13. The prefix pico ______ the base unit ______ times. A. increases 100 A. increases 10 B. increases 1 million B. increases 1 trillion C. decreases 100 C. decreases 10 D. decreases 1 million D. decreases 11. Which of the following units is the largest? A. Kilogram B. Decigram C. Centigram D. Nanogram 1 trillion 14. Which of the following units is not a SI base unit? A. Celsius B. Kilogram C. Meter D. Mole 15. The prefix deci is __________ times smaller than the unit it precedes. Topic 2 – Practice Questions QP 2 A. 10 B. 100 C. 1000 D. 1000000 Grade 09 – CHM 41 16. Write the correct S.I unit for each of the following measurements. Mass ______________________ Length ______________________ Temperature ______________________ 17. A student wrote in his copybook the following paragraph. The class teacher reviewed the work and told the student that the paragraph has some mistakes. Answer questions a and b. A conversion factor is a ratio of different measurements. The measurements in the numerator (on the bottom) is half the measurement in the denominator (on the top). The numerical value and the unit do not change but the actual size changes. a) Underline two wrong words and write the correction for each. b) A student wanted to find the number of seconds in a 50 hour work week. He wrote the answer as follows: 50 s . Is the answer of the student correct? If not, write the correct answer. Topic 2 – Practice Questions QP 3 Grade 09 – CHM 41 18. A student recorded the temperature of a chemical reaction to be 85.0℃. At the end of the reaction the temperature dropped to 60.0℃. Convert the temperatures into Kelvin. (You must show all your work to earn the full mark). 19. Read each of the following statements and identify whether they are True or False. Correct the false statement. a) The prefix kilo is larger than milli. b) A measurement includes a unit only. c) The S.I unit of time is second. d) When using a conversion factor, the actual size changes. Topic 2 – Practice Questions QP 4 Grade 09 – CHM 41 Subtopic 2.2 – Using and Expressing Measurements 5. How many significant figures in 0.004? 1. The radius of a speck is 0.045 mm. The correct value of this number in scientific notation is ____________mm. A. 4.5 × 103 B. 4.5 × 102 C. 4.5 × 10 D. 4.5 × 10 A. One B. Two C. Three D. Infinite 6. How many significant figures in 10.0? 2. The radius of a speck is 0.67 mm. The correct value of this number in scientific notation is ____________mm. A. One B. Two A. 6.7 × 10 C. Three B. 6.7 × 10 D. Four C. 6.7 × 101 D. 6.7 × 102 7. How many significant figures in 1080.? 3. How many significant figures in 2371? A. 1 B. 2 C. 3 D. 4 2.6 × 103 B. 2.6 × 102 C. 2.6 × 10 D. 2.6 × 10 Topic 2 – Practice Questions QP One B. Two C. Three D. Four 8. The sum of the three numbers 8.32, 12.148 and 0.02 to the correct number of significant figures is _______________. 4. The radius of a speck is 0.026 mm. The correct value of this number in scientific notation is ____________mm. A. A. 5 A. 20.488 B. 20.49 C. 20.5 D. 21 Grade 09 – CHM 41 9. The sum of the three numbers 9.765, 12.09 and 0.345 to the correct number of significant figures is _______________. A. 22.200 B. 22.20 C. 22.2 D. 22 13. In which of the following numbers is none of the zero(s) significant? A. 0.00001 B. 0.010 C. 100.0 D. 1065 14. How many significant figures should be retained in the following calculation . . ? . 10. The sum of the three numbers 9.1, 14.156 and 0.07 to the correct number of significant figures is _______________. A. 23.326 A. 1 B. 23.33 B. 2 C. 23.3 C. 3 D. 23 D. 4 11. The sum of the three numbers 7.412, 11.119 and 0.030 to the correct number of significant figures is _______________. 15. The result of the operation . . . reported to the correct number of significant figures is __________________. A. 18.561 B. 18.56 A. 2 C. 18.6 B. 2.5 D. 19 C. 2.46 D. 2.463 12. In which of the following numbers is none of the zeros significant? 16. Which of the following measurements has one significant figure? A. 200.0 B. 1020 A. 0.0004 mL C. 0.020 B. 4000. mL D. 0.002 C. 4.000 mL D. 4.00 mL Topic 2 – Practice Questions QP 6 Grade 09 – CHM 41 17. How many significant figures should be retained in the following . . calculation: ? 17.5/2.40 . A. 1 B. 2 C. 3 D. 4 21. The correct answer for the operation: 456.008 g – 34.008 g is ___________. 1.17 B. 1.176 C. 1.18 D. 1.2 0.0004 mL B. 4000 mL C. 4.000 mL D. 4.44 mL B. 422 C. 422.000 g D. 42.2 A. B. 8.348 m 8.35 m C. 8.3 m D. 8.4 m 23. The number of significant figures in the measurement 103.500 m is __________. 19. Which of the following measurements has four significant figures? A. 422.00 g 22. Subtract 8.998 m – 0.65 m 18. The result of the operation: . . . reported to the correct number of significant figures is __________________. A. A. A. four B. three C. six D. five 24. When 0.90976 cm2 is rounded to three significant figures the correct answer is _____. 20. Which of the following is the correct scientific notation for 202000 g? A. 0.910 cm2 B. 0.91 cm2 A. 2.02000 105 g C. 0.9098 cm2 B. 2.02 105 g D. 0.909 cm2 C. 20.2 104 g D. 2.0200 10-5 g Topic 2 – Practice Questions QP 7 Grade 09 – CHM 41 25. The number of significant figures in 0.00120 cm is __________. Use the following information to answer questions 28 – 30. The following set of measurements is for different objects in a chemistry lab. A. three B. five 11.21 mm C. four 2.410 mm D. two 6.40 mm 22.2 mm 26. A student measured the boiling point of water and found it to be 99.20℃. If the accepted value of the boiling point of water is 100.00℃, then the error is _______. 28. The sum of the above measurements reported to four significant figures is _______. A. 42 mm A. 0.080 B. 42.2 mm B. 0.80 C. 42.22 mm C. 8.00 D. 4222 mm D. 80.0 29. How many of the above measurements has/have four significant figures? 27. The digit 700 has _________ significant figure(s). A. 1 only A. 1 B. 2 only B. 2 C. 3 only C. 7 D. D. 8 All of the measurements have four significant figures 30. The result of dividing 22.2 mm by 6.40 mm has ________ significant figures. __________________________________________________ A. 1 B. 2 C. 3 D. 4 ________________________________________________________ Topic 2 – Practice Questions QP 8 Grade 09 – CHM 41 34. In which of the following numbers is/are the zero(s) insignificant? I. 520. II. 607 III. 18900 IV. 9.000009 Use the following information to answer questions 31 – 33. The following set of measurements is for different objects in a chemistry lab. 12.34 mm 1.530 mm 8.50 mm 11.23 mm 31. The sum of the above measurements reported to four significant figures is ________. A. 3.36 mm B. 33.6 mm C. 33.60 mm D. 336.0mm 1 only B. 2 only C. 3 only D. All of the measurements have three significant figures 1 B. 2 C. 3 D. 4 III only C. II and III only D. III and IV only A. 8.62 10 7 B. 8.62 10 6 C. 8.62 106 D. 8.62 107 A. one B. two C. three D. four 37. The correct scientific notation for 0.000000934 is _______. _____________________________________________________ Topic 2 – Practice Questions QP B. 36. If a student performed the operation below, his answer must be reported to _____ significant figures. . 𝐜 𝐜 . 𝐜 33. The result of dividing 1.530 mm by 8.50 mm has ________ significant figures. A. I only 35. The correct scientific notation for 0.00000862 is _______. 32. How many of the above measurements has/have three significant figures? A. A. 9 A. 9.34 10 7 B. 9.34 10 6 C. 9.34 106 D. 9.34 107 Grade 09 – CHM 41 38. In which of the following numbers is/are the zero(s) not significant? I. 0.0032 II. 0.0004 III. 1.470 IV. 2.502 A. I only B. I and II only C. III and IV only D. I, II and III only 39. If a student performed the operation below, his answer must be reported to _____ significant figures. . 𝐜 .𝐜 . 𝐜 42. Which of the following has “ ” as an uncertain digit? A. 5.265 B. 3.457 C. 5.249 D. 2.556 43. A student was asked to measure the volume of a liquid three times. He recorded three measurements: 68.1 ml, 68.2 mL and 68.0 mL. If the correct measurement was 68.1 mL, the results of the student were _______. A. precise only B. accurate only A. one C. accurate and precise B. two D. inaccurate and imprecise C. three D. four 40. The ________ describes how close a measured value is to an accepted value. 44. The mass of a beaker was measured four times. The masses were 99.997 g, 100.008 g, 100.011 g and 100.005 g. The actual mass is 100.0 g. What can you say about these measurements? A. Accurate and precise B. Inaccurate and imprecise absolute value C. Accurate and imprecise percentage error D. Inaccurate and precise A. accuracy B. precision C. D. 41. The ________ describes how close a series of measurements to one another. A. B. accuracy precision C. absolute value D. percentage error Topic 2 – Practice Questions QP 10 Grade 09 – CHM 41 45. A student was asked to measure the volume of a liquid three times. He recorded three measurements: 72.1 ml, 72.2 mL and 72.0 mL. If the correct measurement was 82.2 mL, the results of the student were _________________. A. precise only B. accurate only C. accurate and precise D. inaccurate and imprecise 46. Ali conducts an experiment five times and gets a solution concentration of 1.9M, 2.1M, 1.8M, 1.9M, and 2.2M. The known concentration of the solution is 3.0M. Which of the following are true about Susan's results? A. Precise only B. Accurate only C. Accurate and precise D. Inaccurate and imprecise 47. A student measured the volume of a liquid three times and found it to be 7.5 mL, 7.4 mL and 7.6 mL. The actual volume of the sample is 7.1 mL. These measurements are ________. A. precise only B. accurate only C. accurate and precise D. inaccurate and imprecise Topic 2 – Practice Questions QP 48. Ahmed is conducting an experiment. His first test gives him a yield of 5.2 grams. His second test gives him a yield of 1.3 grams. His third test gives him a yield of 8.5 grams. On average, his yield is 5.0 grams, which is close to the known yield of 5.1 grams of substance. Which of the following are true about his results? A. Precise only B. Accurate only C. Accurate and precise D. Inaccurate and imprecise 49. A 3 groups of students measure the mass of a product from the same chemical reaction. The groups recorded data of 8.83 g, 8.84 g and 8.82 g. The known mass of the product from that reaction is 8.60g. The group values are _____. A. precise only B. accurate only C. accurate and precise D. inaccurate and imprecise 50. The mass of a beaker was measured and was found to be 47.47 g. Which of the following sets of measurement represents the value with good accuracy and precision? 11 A. 57.47 47.47 37.47 B. 47.46 47.47 47.48 C. 37.47 33.37 57.47 D. 57.46 57.47 57.48 Grade 09 – CHM 41 51. A student measures the mass of different samples of copper nitrate and finds it to be 3.81 g, 3.82 g, 3.79 g and 3.80 g.The actual mass of copper nitrate is 3.92 g.The student’s measurements are _____________ . A. accurate and precise B. accurate and imprecise C. inaccurate and precise D. inaccurate and imprecise 52. Four different students measured the mass of a 1.43 g block four times as shown in the table below. Trial 1 Trial 2 Trial 3 53. The length of a metal piece was measured to be 12.6 cm. Which of the following sets of measurement represents the values with good accuracy and precision? 1.43 g 1.44 g 1.42 g 1.45 g Student X 1.43 g 1.50 g 1.46 g 1.44 g Student Y 1.54 g 1.56 g 1.58 g 1.50 g Student Z 0.86 g 1.24 g 1.52 g 1.42 g The results indicate that the data collected by student ____ has the greatest accuracy and precision. 13.2 13.4 13.3 B. 13.2 11.5 12.1 C. 12.5 12.6 12.7 D. 12.1 11.9 12.9 54. The volume of a liquid was measured and it was found to be 15.5 mL. Which of the following sets of measurement represents the values with good accuracy and precision? Trial 4 Student W A. A. 16.2 16.3 16.8 16.9 B. 12.3 12.1 12.4 12.2 C. 15.2 15.3 15.4 15.6 D. 17.1 17.3 17.4 17.6 55. Four students measured the mass of a sample of zinc sulfate several times and found it to be 4.81 g, 4.82 g, 4.79 g and 4.80 g.The actual mass of zinc sulfate was 4.81 g.The student’s measurements were _____________. A. W A. accurate and precise B. X B. accurate and imprecise C. Y C. inaccurate and precise D. Z D. inaccurate and imprecise Topic 2 – Practice Questions QP 12 Grade 09 – CHM 41 68. A technician experimentally determined the boiling point of octane to be 124.1 ℃. The actual boiling point of octane is 125.7 ℃. The error is ______. A. 1.6 ℃ B. 1.3 ℃ C. 1.3 ℃ D. 1.6 ℃ 69. A student measures the depth of a swimming pool to be 4.04 m at its deepest end. The accepted value is .00 m. The student’s percent error is _______. A. 0.04 % B. 0.1 % C. 1% D. 4% 72. A technician experimentally determined the mass of a beaker to be 120.5 g. The actual mass of the beaker is 117.9 g. The error is ______ g. A. 2.6 B. 2.00 C. 2.90 D. 29.0 2.3 D. 2.6 B. Systematic error C. Random error D. Gross error 74. Which of the following graphs represent(s) a direct relationship? I. II. 71. The accepted value of a length measurement is 200 cm, and the experimental value is 198 cm. The percentage error of this measurement is ________. A. 2% B. C. 73. Which of the following errors is caused by poor calibration of instruments? A. Multidirectional error 70. A chemist measured the amount of caffeine in a new energy drink and found it to be 87.10 mg. If the accepted amount of caffeine was 84.20 mg, then the error is _______. A. 0.29 B. 2.3 III. 1% C. 1% D. 2% Topic 2 – Practice Questions QP 15 A. I only B. II only C. III only D. I and III only Grade 09 – CHM 41 79. Indicate the number of significant figures in each of the following measurements. a) 32.25 ______________ b) 0.00005 ______________ c) 1000 ______________ d) 98007 ______________ e) 605041 ______________ f) 12 students ______________ g) 2000 ______________ h) 9.00 ______________ i) 200090. ______________ j) 246.22 ______________ k) 1.0006 ______________ l) 50000 ______________ m) 320024 ______________ n) 123 m ______________ o) 40,506 mm ______________ p) 9.8000 x 104 m ______________ q) 22 meter sticks ______________ r) 0.070 80 m ______________ s) 98,000 m ______________ t) 345.21 ______________ u) 0.000341 ______________ v) 1400 ______________ Topic 2 – Practice Questions QP 18 Grade 09 – CHM 41 80. Perform the following operations. Make sure that your answers have the correct number of significant figures and unit. a) 3.20 cm × 150. cm b) (2.0 × 10 c) (1.20 4 m) × (1.00 107 m) d) 148.576 g 10 2 m) (1.20 10 2 m) 35.41 g e) 13.00 m – 0.54 m f) (1.0 10 5 m) × (3.00 10 4 m) g) 36.47 cm + 2.721 cm + 15.1 cm h) (1.20 i) 8.0 j) 3.633 107 m) (1.20 10 2 m) 4.0 10 10 10 k) 22.34 km 2.1 10 1.3 km Topic 2 – Practice Questions QP 19 Grade 09 – CHM 41 l) 1.2 m 3.11 m 81. Use your knowledge about scientific measurement and units of measurement to answer questions a – e. a) Rank the following units in an increasing order of their length. nanometer, millimeter, decimeter and picometer b) Write a conversion factor that can be used to convert 3 days to minutes. c) A substance has a temperature of 20 ℃. This temperature is equivalent to _________K. d) The SI unit of mass is ____________. e) The prefix centi is 100 times ___________ than the unit it precedes. 82. Three students carried out several weightings of a copper cylinder, each using a different balance. Describe the accuracy and precision of each student’s measurements if the correct mass of the cylinder is 47.32 g. Weighing 1 Weighing 2 Weighing 3 Weighing 4 Mass of Cylinder (g) Student 1 Student 2 47.13 47.45 47.94 47.39 46.83 47.42 47.47 47.41 Student 1: ______________________________ Student 2: ______________________________ Student 3: ______________________________ Topic 2 – Practice Questions QP 20 Student 3 47.95 47.91 47.89 47.93 Grade 09 – CHM 41 83. Read the following passage then answer questions a – e. In the past, people used some human body parts as units of measurement for length such as arms, fingers and legs. Nowadays, an international system of units, SI units, is widely used to express mea remen of leng h ma empera re ime e c Prefi e are added o SI base units to indicate larger or smaller quantities like mega, kilo and deci. a) Consider the following units: micrometer, meter, kilometer and centimeter. o The largest unit is ________________________. o The smallest unit is ________________________. b) The prefix nano changes the SI base unit by a factor of ____________. c) Identify the SI base unit for each of the following quantities. o Temperature ________________ o Amount of substance ________________ d) A solution has a temperature of 333 Kelvin. This temperature is equivalent to _________℃. e) Write a conversion factor that can be used to convert 10 years to hours. Topic 2 – Practice Questions QP 21 Grade 09 – CHM 41 84. Four students measured the mass of a 5.43 g block of metal three times. The results were recorded in the table below. Answer questions a - e. Trial 1 Trial 2 Trial 3 Student W 5.43 g 5.44 g 5.42 g Student X 5.43 g 5.40 g 6.43 g Student Y 5.54 g 5.56 g 6.41 g Student Z 6.86 g 6.86 g 6.87 g a) Which student had the greatest precision but worst accuracy? b) Which student had the greatest precision and greatest accuracy? c) Which student had systematic error in all his results? d) Which student had the highest precision? e) Which student had the greatest accuracy? Topic 2 – Practice Questions QP 22 Grade 09 – CHM 41 85. Two students (X and Y) measured the diameter of a gold coin four times. The results were recorded in the table below. The actual diameter of the coin is 28.0 mm. Answer questions a – c. Student Trial 1 Trial 2 Trial 3 Trial 4 Student X 28.1 mm 28.0 mm 28.0 mm 27.9 mm Student Y 27.3 mm 27.3 mm 27.4 mm 27.4 mm a) Which student (X or Y) had measurements that are precise but not accurate? b) Which student (X or Y) had measurements precise and accurate? c) Which student (X or Y) had measurements with greater accuracy? d) For student Y, calculate the percentage error in trial 1. Topic 2 – Practice Questions QP 23 Grade 09 – CHM 41 d) Which of the following graphs (W, X, Y or Z) can be used to represent the data collected in this experiment? Circle the correct graph. 89. A weather forecast report predicted 20.0 mm of rain in one of the northern emirates. The actual amount of rain was 25.0 mm. What is the percent error of the weather forecast report? 90. A scientist reported the age of an ice layer at 85 m as 525 years. The accepted value is 500 years. What is the percent error of the scientist’s value? Topic 2 – Practice Questions QP 26 Grade 09 – CHM 41