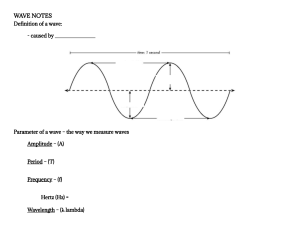
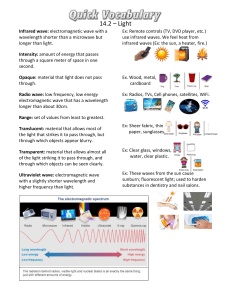
ELECTROMAGNETIC SPECTRUM Week 1 OBJECTIVES: 1.Trace the development of electromagnetic wave theory. 2.Define electromagnetic waves. 3. Describe the transmission and propagation of electromagnetic waves. 4. Discuss the properties of EM waves. 5. Solve problems involving wavelength, frequency, and energy of an electromagnetic wave. 6. Compare the relative wavelengths, frequencies, and energies of the different regions of EM waves. What is a wave ? A wave can be thought of as a disturbance or oscillation that travels through space-time, accompanied by a transfer of energy. • Kinds of waves Kinds of waves 1. Mechanical wave - is a wave that is an oscillation of matter and is responsible for the transfer of energy through a medium. Where does the energy come from in the water wave pictured above? Answer: The energy comes from the falling droplets of water, which have kinetic energy because of their motion. Medium The matter through which the wave travels is called the medium. How do the particles of the medium move when a wave passes through them? • The particles of the medium just vibrate in place. • As they vibrate, they pass the energy of the disturbance to the particles next to them, which pass the energy to the particles next to them, and so on. • Particles of the medium don’t actually travel along with the wave. Only the energy of the wave travels through the medium. Types of Mechanical waves Types of Mechanical waves a. Transverse Wave When the movement of the particles is at right angles or perpendicular to the motion of the energy, then this type of wave is known as Transverse wave. Ex. light Examples of Transverse waves Light Vibrations on a string Ripples on the surface of water. Types of Mechanical waves b. Longitudinal Wave In this type of wave, the movement of the particle are parallel to the motion of the energy Examples of Longitudinal waves Sound waves Compressions moving along a slinky P-waves Kinds of waves 2. Matter wave - All matter exhibits a wave-like behavior. For example, a beam of electrons can be diffracted just like a beam of light or a water wave. - The concept that matter behaves like a wave was proposed by French physicist Louis de Broglie 3. Electromagnetic Wave - considered to be both of electric and magnetic in nature - contains an electric field and a magnetic field 3. Electromagnetic Wave - All electromagnetic waves can travel through a vacuum at the same speed - do not need a medium to travel. Speed of light: 3.0 x 108 m/s Characteristics of a wave Characteristics of a wave 1. Amplitude ● ● The maximum position moved by a point on a wave measured from its equilibrium position The height of a wave Characteristics of a wave 2. Wavelength λ ● The distance between two consecutive crest or two consecutive trough of a wave Characteristics of a wave 3. Frequency (f) ● ● higher frequency = higher energy The number of waves that pass a fixed point in a given amount of time The SI unit for wave frequency is the hertz (Hz) The wave speed, frequency, and wavelength are related by the following equation: Where: c=λf c = speed of light (3.0 x 108 m/s) f = frequency in Hertz λ = wavelength in meters. Sample problem 1 What is the frequency of the radiowave with a wavelength of 25 m? Sample problem 1 What is the frequency of the radiowave with a wavelength of 25 m? Given c = 3 x 108 m/s λ = 25 m f = ? Formula c = f λ λ λ c λ = f c or f = λ Sample problem 1 What is the frequency of the radiowave with a wavelength of 25 m? Given 108 c = 3 x λ = 25 m f = ? m/s Formula c f = λ Solution f = 3 x 108 m/s 25 m f = 1.2 x 107 Hz Sample problem 2 Calculate the frequency of a wave if the wavelength decreases from 25 m to 10 m. Sample problem 1 Calculate the frequency of a wave if the wavelength decreases from 25 m to 10 m. Given c = 3 x 108 m/s λ = 10 m f = ? Formula c = f λ λ λ c λ = f c or f = λ Sample problem 1 Calculate the frequency of a wave if the wavelength decreases from 25 m to 10 m. Given 108 c = 3 x λ = 10 m f = ? m/s Formula c f = λ Solution f = 3 x 108 m/s 10 m f = 3 x 107 Hz Sample Problem 1 λ = 25 m Sample Problem 2 λ = 10 m What did you observe with the wavelengths? “The wavelength of the wave decreases” f = 1. 2 x 107 Hz f = 3 x 107 Hz What did you observe with the frequencies? “The frequency of the wave increases” 3. Kim made a wave in a spring by pushing and pulling on one end. The wavelength is 0.1 m, and the wave frequency is 2 hertz. What is the speed of the wave? What is the relationship of the wavelength to the frequency? λ f The wavelength is inversely proportional to the frequency of the wave As the wavelength decreases, the frequency increases and vice versa Solve the following: 1. What is the frequency of the green light that has a wavelength of 5.5 x 10-7m ? 2. What is the wavelength of a microwave that has a frequency of 4.2 x 108 Hz? 3. A wave is traveling at a speed of 2 m/s and has a frequency of 2 Hz. What is its wavelength? Electromagnetic Spectrum Electromagnetic Spectrum Seatwork no. 1 1. Arrange the following according to decreasing wavelength. X-ray Radio wave Gamma ray Answer: Radio wave X-ray Gamma ray Seatwork no. 1 2. Arrange the following according to increasing wavelength. Infrared Microwave Visible light Answer: Visible light Infrared Microwave Seatwork no. 1 3. Arrange the following according to increasing frequency. Infrared Microwave Visible light Answer: Microwave Infrared Visible light Seatwork no. 1 3. Arrange the following according to decreasing frequency. Ultraviolet ray Gamma ray Microwave Answer: Gamma ray Ultraviolet ray Microwave Seatwork no. 1 3. Arrange the following according to decreasing energy. Radio wave Ultraviolet ray Infrared Answer: Ultraviolet ray Infrared Radio wave 1. Showed how a current carrying wire behaves like a magnet a. Heinrich Hertz 2. Showed experimental evidence of electromagnetic waves and their link to light. b. Hans Christian Oersted 3. Contributed to developing equations that showed the relationship of electricity and magnetism. c. André Marie Ampère 4. Formulated the principle behind electromagnetic induction d. James Maxwell 5. Demonstrated the magnetic effect based on the direction of current e. Michael Faraday Seat Work no. 1 https://www.du cksters.com/sc ience/quiz/wav es_questions.p hp II. Solve the following: 1) A wave has frequency of 50 Hz and a wavelength of 10 m. What is the speed of the wave? 2) The speed of a wave is 65 m/sec. If the wavelength of the wave is 0.8 meters, what is the frequency of the wave? I. 1. Arrange the following according to increasing energy. microwave X-ray Ultraviolet ray Answer: microwave Ultraviolet ray X-ray Seatwork no. 1 2. Arrange the following according to increasing wavelength. microwave infrared Gamma ray Answer: Gamma ray infrared microwave Seatwork no. 1 3. Arrange the following according to decreasing wavelength. Ultraviolet ray Visible light Gamma ray Answer: Visible light Ultraviolet ray Gamma ray Photons - are bundles of wave energy. The energy of a photon is given by the equation: E=hf Where: E= Energy h= Planck’s Constant (6.63 x 10-34 joules second) f= frequency of the EM wave Example Problems: (Assume that the waves propagate in a vacuum.) 1. A photon has a frequency (f) of 2.68 x 106 Hz. Calculate its energy 2. Calculate the energy (E) of a photon of light with a frequency (f) of 6.165 x 1014 Hz. Seatworks: Answer: WHAT TO DO (PROCEDURE) PART B. SOLVING PROBLEMS INVOLVING WAVELENGTH, FREQUENCY, AND ENERGY OF AN ELECTROMAGNETIC WAVE.