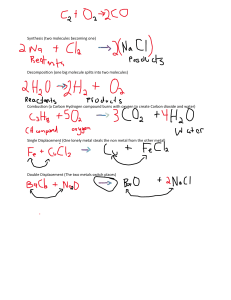
Contents 1. 1 Notes 1. 1.1 Metals in the Periodic Table 2. 1.2 Properties 1. 1.2.1 Extremes in Metals 2. 1.2.2 Metallic properties 3. 1.3 Alloys 4. 1.4 Reactivity series of metals 5. 1.5 Stability of metal compounds 6. 1.6 Displacement power of metals 1. 1.6.1 Displacement of oxides 2. 1.6.2 Displacement from solutions 3. 1.6.3 Reaction of Metal Oxides with Carbon 4. 1.6.4 Reaction of Metal Oxides with Hydrogen 5. 1.6.5 Decomposition of Metal Carbonates 7. 1.7 Extraction of Metals 1. 1.7.1 Uses of Metals 2. 1.7.2 Recycling Metals 8. 1.8 Iron 9. 1.9 Steel 10. 1.10 Rusting 2. 2 MCQ Questions 1. 2.1 Answers 2. 2.2 Structured Questions and Worked Solutions Notes Metals in the Periodic Table Mainly in Group I, Group II, and the Transition Block those near the staircase line Properties 1. High density, melting point and boiling point due to close packing of the atoms in metals strong forces between these atoms high density except sodium high melting and boiling points except mercury and sodium 2. Malleable and ductile when a force is applied to a metal, the atoms can slide over one another malleable: can be bent and beaten into different shapes ductile: can be stretched to form wires 3. Thermal conductivity heat energy can be transferred from one atom to another by vibration as the atoms are very close together the outermost electrons also help to conduct heat 4. Electrical conductivity when a metal is connected to a circuit, the free outermost electrons move towards the positive terminal to replace them, more electrons are fed into the metal from the negative terminal Metals always from positive ions Extremes in Metals Lightest: Lithium Heaviest: Osmium Most brittle: Manganese and chromium Lowest melting point: Mercury Highest melting point: Tungsten Most expensive: Platinum Rarest: Rhodium Most abundant: Aluminium Metallic properties Metals have high: - density: high mass per unit volume - tensile strength: high strength of the metal under stress - durability: resistant to corrosion - malleability: ability to be made into sheets - ductility: ability to be made into wires - thermal conductivity: ability to conduct heat - electrical conductivity: ability to conduct electricity - sonority: ability to produce sound when struck Alloys a mixture of metallic elements or metallic with non-metallic. Pure metals are weak as the layers of atoms slide over each other easily. in alloy of 2 metals, they have different sizes of atoms so this distrupts the orderly layer of atoms making it difficult for atoms to slide over. Eg of alloys Steel: iron and carbon bronze: copper and tin brass: copper and zinc duralumin: aluminium, copper, magnesium Uses of duralumin: it is light but strong and durable so used for aircraft parts, greenhouse frames, overhead cables, curtain walling in high-rise buildings etc. pewter: tin and lead Uses of solder: mixture of tin and lead, has a much lower melting point than either of its components so more easily fusible --- suitable for welding electrical wire together Uses of stainless steel: is an alloy of iron containing chromium or nickel. Is the most expensive way applications for: cutleries medical instruments kitchen sinks steel objects in chemical factories and oil refineries Reactivity series of metals Reactive metals tend to form positive ions easily, by losing electrons and forming compounds unreactive metals prefer to remain in uncombined form, as the element itself the order of reactivity is worked out from the metal's reaction (if any) with water or steam and acids if there is a reaction, the metal displaces hydrogen Metal + hydrogen ion ---> metal ion + hydrogen gas Metal potassium sodium calcium Metal with water/steam react with cold water M(s) + 2H2O(l) --> MOH(aq) + H2(g) Metal + Water --> Metal Hydroxide + Hydrogen Metal with acid violent reaction with dilute acids M(s) + 2HCl(aq) --> MCl2(aq) + H2(g) Metal + Acid --> Metal Chloride + Hydrogen magnesium aluminium zinc iron react with steam M(s) + 2H2O(g) --> MO(s) + H2(g) Metal + Water --> Metal Oxide + Hydrogen react with dilute acids with decreasing ease M(s) + 2HCl(aq) --> MCl2(aq) + H2(g) Metal + Acid --> Metal Chloride + Hydrogen lead hydrogen do not react with water or steam react with dilute acids with decreasing ease copper mercury silver platinum do not react with water or steam react only with concentrated acids In the reactivity series, metals at the top, like potassium and sodium, react violently with cold water. Hence, they are stored under the surface of oil to prevent water vapour in the atmosphere from reacting with them eg. 2Na + 2H2O ---> 2NaOH + H2 Down the series, the reactivity of the metal decreases. Magnesium will react only with steam, and for metals below iron there is no reaction with either cold water or steam. eg. Mg + H2O ---> MgO + H2 With dilute hydrochloric acid, the metals at the top of the series react very violently. As we go down, the metals react less vigorously. Aluminium, although above iron and zinc, reacts more slowly because of a protective oxide coat on its surface. eg. Fe + 2HCl ---> FeCl2 + H2 Below lead, there is no reaction with steam or with dilute acids and so hydrogen is never displaced. Hence its position in the series. The metals below hydrogen will react only with concentrated acids which are capable of oxidising the metal first to its oxide. Such acids are concentrated nitric or sulphuric acids eg. Cu + 4HNO3 ---> Cu(NO3)2 + 2NO2 +2H2O Stability of metal compounds Compounds of metals high up in the reactivity series are stable and not easily decomposed by heating. Compounds of metals low down in the series are unstable, and are often decomposed by heating, or are easily reduced. The oxides of metals above zinc in the series can only be reduced to the metal by using electrolysis. At cathode, reduction occurs + Al3 + 3e ---> Al The oxides below can be reduced with reducing agents like carbon or hydrogen, except zinc oxide which cannot be reduced by action of hydrogen ZnO + C --> Zn + CO CuO + H2 --> Cu + H2O Metal potassium sodium Oxide electrolytic reduction calcium magnesium aluminium electrolytic reduction zinc iron lead copper reduced by heating with carbon mercury silver platinum reduced by heating alone Hydroxide stable to heat Carbonate stable to heat Nitrate decompose to nitrite and oxygen decompose to decompose to decompose to metal oxide metal oxide and metal oxide, and steam on carbon dioxide nitrogen dioxide heating gas on heating and oxygen on heating decompose decompose to decompose to to metal oxide metal oxide and metal oxide, and steam on carbon dioxide nitrogen dioxide heating gas on heating and oxygen on heating unstable, do unstable, do decompose to not exist not exist metal, oxygen and nitrogen dioxide gas on heating Down the series, reduction becomes easier because the metals prefer to exist as atoms, as opposed to ions For metal oxides like mercury(II) oxide, no reducing agent is needed - just heating alone 2HgO --> 2Hg + O2 Hydroxides of the metals calcium and below decompose to their corresponding oxide and give off steam, on heating. This can be confirmed by using anhydrous copper(II) sulphate which turns white to blue with steam Ca(OH)2 ---> CaO + H2O Similarly, most carbonates, except sodium and potassium carbonates, undergo thermal decomposition again to a metal oxide, but this time giving off carbon dioxide gas. This can be confirmed by bubbling the gas through limewater, which turns milky with carbon dioxide PbCO3 ---> PbO + CO2 Nitrates also decompose on heating, but the stable ones at the top of the series only decompose as far as the nitrite (nitrite(III)), giving off oxygen gas. This can be identified by the gas relighting a glowing splinter 2KNO3 ---> 2KNO2 + O2 The majority of nitrates decompose to the metal oxide, giving off brown fumes of nitrogen dioxide as well as oxygen gas. 2Mg(NO3)2 ---> 2MgO + 4NO2 + O2 The unstable nitrates at the bottom of the reactivity series decompose all the way to the metal itself 2AgNO3 ---> 2Ag + 2NO2 + O2 Displacement power of metals Displacement reaction is the displacement of ions of metal from compounds of metals lower in reactivity series by metals higher in reactivity series. E.g. Magnesium displaces copper(II) chloride Mg(s) + CuCl2(aq) -> MgCl2(aq) + Cu(s) For observation, we’ll see silver magnesium metal coated with brown copper metal Displacement is due to Mg atoms transfer electrons to Cu2+ ions forming Cu atoms. 2+ - Mg(s) → Mg (aq) + 2e 2+ Cu (aq) + 2e → Cu(s) Loss of electrons is due to it’s less reactive as less reactive metal has higher chance of losing electrons. That’s why when Mg is placed in KCl, no reaction occurs. Mg(s) + KCl2(aq) --> No reaction Displacement of oxides The Thermit Reaction is an example of displacement of oxides. Iron(III) oxide and aluminium powder are heated in a crucible, with a magnesium fuse to start the reaction. The aluminium is more reactive, and takes the oxygen from the iron oxide, leaving molten iron at the bottom of the crucible. Fe2O3 + 2Al ---> Al2O3 + 2Fe This reaction is called the Thermit Reaction as it produces large quantities of heat. It has been used to weld railway lines in remote areas where normal welding techniques are not possible. Displacement from solutions In general, the more reactive metal goes into solution displacing the less reactive. For eg, if iron filings were slowly added, with stirring, to a blue solution of copper(II) sulphate, the blue color would fade and become faintly greenish. This is because the copper has been pushed out, and is left as pink copper metal, while the iron has gone into solution as green iron(II) sulphate CuSO4 + Fe ---> FeSO4 + Cu In displacement reactions, the powder form is used as powders have a greater surface area and so will react more quickly. Using ionic equations to show displacements: 2+ 2+ Cu + Fe ---> Fe + Cu Reaction of Metal Oxides with Carbon The lower the position of metal in reactivity series, the easier for carbon to remove oxygen from metal oxide by heating. At higher position, stronger heat is needed. E.g. CuO reacts with C can be reduced by bunsen burner flame temperature CuO(s) + C(s) --> Cu(s) + CO2(g) For iron oxide to be reduced, it needs very high temperature. Reaction of Metal Oxides with Hydrogen The lower position of metal in reactivity series, the easier hydrogen remove oxygen from metal oxide by heating. At higher position, stronger heat is needed. E.g. PbO reacts with H2 can be reduced by bunsen burner flame temperature PbO(s) + H2(g) --> Pb(s) + H2O(l) Decomposition of Metal Carbonates The lower position of metal in reactivity series, the easier hydrogen remove oxygen from metal oxide by heating. At higher position, stronger heat is needed. E.g. CuCO3 reacts decomposes by heat of bunsen burner flame temperature CuCO3(s) --> Cu(s) + CO2(g) Extraction of Metals Metals from Rocks Minerals – elements/compounds that make up rocks Metal ore – rock containing metal Extracting these metals Metal ores are removed from ground. The ores contain useful and unwanted materials. Unwanted materials are separated to obtain concentrated mineral. Metal is extracted from the mineral. Occurrence of Metals Metal ores are compounds, usually as: Metal oxides – metal + oxygen, eg: Al2O3 Metal sulphides – metal + sulphur, eg: HgS Metal carbonates – metal + carbon + oxygen, eg: MgCO3 Least Reactive – easiest to extract; extracted by physical methods Less Rective – harder to extract than least reactive; by blast furnace; usually occur as compounds of oxides or sulphides. Most Reactive – hardest to extract – strong bonds in compounds; by electrolysis – decomposing compounds with electricity. Uses of Metals The choice of metals over another depends on 3 factors: Physical properties (e.g. melting point, strength, density, conductivity) Chemical properties (e.g. resists corrosion) Cost Recycling Metals There are many iron on the surface but copper and tin are seriously reducing. High temperatures and pressures and greater depth increases hazards that prevent mining up to the lower part of crust, although there are more metals further down Ways to conserve metals Use alternative materials to replace the use of iron (e.g. use of plastic pipes instead of iron, use of glass bottles for soft drinks instead of aluminium) Recycle unused metals by melting them to produce new blocks of clean metal Advantages of recycling metals Recyling helps conserving metals, especially valuables such as gold and platinum. E.g. used computer parts processed to extract gold used for electrical contacts of processors and memory chips Recycling saves the cost of extracting new metals Recycling benefits environment, e.g. if there is a car wasteland, it causes eyesore Recycling metals can damage the environment by smelting process which sends a lot of fumes into the air Cost to separate metals from waste is high. E.g. separat metals in alloys is hard Transport costs for collecting scrap metal is high, e.g. trucks should be used People are not interested in depositing their used materials in recycling bins Iron Steel Iron is extracted from the iron ore haematite, Fe2O3 Iron is extracted from the oxide in a blast furnace Iron made from blast furnace is not good as: it contains impurities which makes it brittle (can break easily) it cannot be bent or stretched Most iron is converted into steel which is an alloy of iron and carbon with small amounts of other elements. Advantages of steel: it is strong and tough it can be bent and stretched without shattering Making Steel: Impurities of iron is removed by blowing oxygen into molten iron to change the impurities into oxides. They are then combined with CaO and removed as slag. Carbon and other metals are added in certain amount to make steel. Different Types of Steel: Mild steel – is a low carbon steel with 0.25% carbon It is strong and quite malleable. It is used for car bodies, ships, railway lines and steel rods to reinforce concrete Hard steel – is a high-carbon steel with about 1% carbon It is harder than mild steel and less malleable. It is used to make tools Stainless steel – is iron with large amounts of chromium and nickel It is hard, shiny and doesn’t rust. It is used to make cutleries, medical instrument and pipes in chemical industries. Rusting Rusting – corrosion of iron and steel Rust – brown solid product formed during rusting Rust is hydrated iron(III) oxide Fe2O3.xH2O where water molecules varies. Conditions for rusting Tubes A B C After a few days, only nail in tube A rust. This shows that air and water is needed for rust. In boiled water, the nail doesn’t rust in B as boiled water removes dissolved air while in C, CaCl keeps air dry so there’s no water. Preventing Rusting Surface protection: covers metal with a layer of substance Paint Grease or oil (also help to lubricate) Plastic Metal Plating – covering metal with thin layer of another metal (e.g. tin, chromium, silver) Advantage – These methods are cheap (except metal plating) Disadvantage – If the layer is broken, air and water an reach metal to rust Sacrificial protection to sacrifice more reactive metal to corrode with water and air by layering it over less reactive metal (e.g. iron covered by magnesium). If layer is broken, water & air reach underneath layer, overlying metal still protect it. Applications: Galvanised Iron – is steel coated with zinc, usually used on roofs. Protecting ships – blocks of zinc are attached to hulls to corrode instead of steel which is the ship metal. Underground steel pipes – these are attached to magnesium block using insulated copper cables. Magnesium corrodes first than steel. Use of stainless steel MCQ Questions 1. Caesium is a metal that is more reactive than aluminium. Which reaction would produce caesium? a. electrolysing aqueous caesium chloride b. electrolysing molten caesium chloride c. heating caesium carbonate d. heating caesium oxide with carbon 2. Which of the following processes does not result in the formation of both carbon dioxide and water? a. addition of a dilute acid to a carbonate b. burning ethanol c. burning methane d. heating crystals of hydrated sodium carbonate 3. Which element is always present with iron in mild steel? a. aluminium b. carbon c. chromium d. nickel 4. Hydrogen gas will reduce a. calcium oxide b. silver oxide c. magnesium oxide d. potassium oxide 5. Which oxide can be reduced to the metal using carbon a. calcium oxide b. magnesium oxide c. sodium oxide d. zinc oxide 6. Which substance removes impurities from iron ore in the blast furnace? a. carbon b. limestone c. sand d. slag 2+ 2+ 7. An excess of iron filings is added to a solution containing a mixture of the ions Mg , Ca , 2+ + Cu and Ag . Which 2 metals will be displaced from this solution? a. calcium and copper b. calcium and magnesium c. copper and silver d. magnesium and silver 8. What reacts with hydrochloric acid to give hydrogen? a. ammonia b. iron c. silver d. sodium hydroxide 9. Why does the color of aqueous potassium bromide change when chlorine gas is bubbled into it? a. a compound is formed between chlorine and bromine b. a solution of potassium chloride is formed c. the chlorine oxidises bromide ions to bromine d. the potassium bromide is reduced 10. Which carbonate decomposes on heating to give a black solid and a colourless gas? a. calcium carbonate b. copper(II) carbonate c. sodium carbonate d. zinc carbonate 11. Which substance is not an essential raw material in the extraction of iron in a blast furnace? a. air b. coke c. limestone d. sand 12. Which element reacts with oxygen to form a compound that is a gas at room temperature? a. magnesium b. hydrogen c. copper d. carbon 13. Caesium is a metal that is more reactive than aluminium. Which reaction would produce caesium? a. electrolysing aqueous caesium chloride b. electrolysing molten caesium chloride c. heating caesium carbonate d. heating caesium oxide with carbon 14. What is a disadvantage of recycling metals? a. collection and transportation costs money b. metal ores are a finite resource c. most metals corrode slowly in the environment d. scrap metal melts when heated 15. Which of the following processes does not result in the formation of both carbon dioxide and water? a. addition of a dilute acid to a carbonate b. burning ethanol c. burning methane d. heating crystals of hydrated sodium carbonate 16. Hydrogen gas will reduce a. calcium oxide b. silver oxide c. magnesium oxide d. potassium oxide 17. Which element is always present with iron in mild steel? a. aluminium b carbon c. chromium d. nickel 18. Which oxide can be reduced to the metal using carbon? a. calcium oxide b. magnesium oxide c. sodium oxide d. zinc oxide 19. Which substance removes impurities from iron ore in the blast furnace? a. carbon b. limestone c. sand d. slag 20. What reacts with hydrochloric acid to give hydrogen? a. ammonia b. iron c. silver d. sodium hydroxide 21. Which carbonate decomposes on heating to give a black solid and a colorless gas? a. calcium carbonate b. copper(II) carbonate c. sodium carbonate d. zinc carbonate 22. Which substance is not an essential raw material in the extraction of iron in a blast furnace? a. air b. coke c. limestone d. sand 23. Which element reacts with oxygen to form a compound that is a gas at room temperature? a. magnesium b. hydrogen c. copper d. carbon 24. A sample of air is slowly passed through aqueous sodium hydroxide and then over heated copper. Which gases are removed by this process? a. carbon dioxide and water vapour b. carbon dioxide and oxygen c. nitrogen and oxygen d. nitrogen and water vapour 25. When heated, solid X gives off a gas which turns limewater milky. The residue reacts with dilute acid and also with aqueous alkali. What is X? a. copper(II) carbonate b. magnesium carbonate c. sodium carbonate d. zinc carbonate 26. An element is burned in an excess of oxygen. Which statement about the oxide formed is always correct? a. it is a crystalline solid b. it is greater in mass than the element c. it is soluble in water d. it is white in color 27. Which substance can be reduced by carbon? a. aluminium oxide b. calcium carbonate c. iron(III) oxide d. magnesium oxide 28. Which of the following is a typical property of transition metals? a. they form colored compounds b. they have low densities c. they have low melting points d. they react with cold water to give hydrogen 29. What happens when zinc is placed in aqueous copper(II) sulphate? a. copper atoms are oxidised b. zinc atoms are oxidised c. copper ions are oxidised d. zinc ions are oxidised 30. Which substance does not need air as a raw material for its manufacture? a. ammonia b. iron c. sodium d. sulphuric acid 31. Which of the following is not a use of silicon or its compounds? a. making fire-resistant plastics b. making glass c. making polishes d. making smokeless fuel 32. Compound X reacts with some metals to liberate hydrogen and is used to make fertilisers. It gives a white precipitate when added to aqueous barium nitrate. What is X? a. ammonium sulphate b. hydrochloric acid c. potassium nitrate d. sulphuric acid 33. Which industrial process uses iron as a catalyst? a. making ammonia from nitrogen and hydrogen b. making ethanol from ethene and steam c. making steel d. making sulphur trioxide from sulphur dioxide and oxygen 34. Which pair of elements will combine to form an ionic compound? a. carbon and chlorine b. fluorine and sodium c. hydrogen and oxygen d. oxygen and carbon 35. How does the mass of a sample of copper(II) oxide change when it is heated in hydrogen and in oxygen? a b c d mass after heating in hydrogen decreases decreases unchanged unchanged mass after heating in oxygen decreases unchanged decreases unchanged 36. Sodium is a metal. Using only this information, what can be deduced about sodium? a. it has a low melting point b. it is a conductor of electricity c. it is less dense than water d. it is very reactive 37. Which substance reacts with water to form a soluble compound and an insoluble gas? a. ammonium sulfate b. caesium c. calcium carbonate copper 38. Which compound does not give off a gas when heated? a. hydrated copper(II) sulfate b. hydrate sodium carbonate c. magnesium carbonate d. sodium carbonate 39. Which metal should be used in the sacrificial protection of the hull of a boat made from iron? a. calcium b. copper c. lead d. zinc 40. A coil of clean copper wire is suspended in a beaker of aqueous silver nitrate. Crystals of silver are deposited on the copper wire. Which statement is not correct? a. the copper is oxidised b. the solution turns blue c. the total mass of the crystals of silver increases gradually d. the total number of positive ions in the solution is unchanged 41. In the manufacture of iron by the blast furnace, which are the main gases that escape from the top of the blast furnace? a. carbon monoxide, carbon dioxide, hydrogen b. nitrogen, carbon dioxide, carbon monoxide c. nitrogen, oxygen, steam d. oxygen, carbon dioxide, sulfur dioxide 42. When heated, solid X gives off a gas. When this gas is bubbled through limewater, a white precipitate is formed. The residue after heating solid X reacts with dilute acid and also with aqueous alkali. What is X? a. copper(II) carbonate b. magnesium carbonate c. sodium carbonate d. zinc carbonate 43. The information below concerns 3 elements X, Y, and Z. X: Its oxide is decomposed by heat to the element. Y: Its carbonate is not decomposed by heat. Z: Its oxide is not decomposed by heat but its carbonate decomposes. In order of decreasing reactivity, the 3 elements should be arranged as: a. Y Z X b. X Y Z c. Y X Z d. X Z Y Answers 1. b 2. d 3. b 4. b 5. d 6. b 7. c 8. b 9. c 10. b 11. d 12. d 13. b 14. a 15. d 16. b 17. b 18. d 19. b 20. b 21. b 22. d 23. d 24. b 25. d 26. b 27. c 28. a 29. b 30. c (sodium is obtained through electrolysis) 31. d 32. d 33. a 34. b 35. b 36. b 37. b 38. c 39. d 40. b 41. b 42. d 43. a Structured Questions and Worked Solutions 1a. Under what conditions does water react with i. sodium ii. magnesium In each case, name the products formed. b. Water supplies are obtained from rivers, boreholes and reservoirs. The water must be treated before use. Describe and explain the two main processes in the purification of water supplies. c. Water supplies that have passed through iron pipes contain iron(II) ions, Fe 3+ Fe . 2+ and iron(III) ions, In the presence of air, iron(II) ions are slowly changes to iron(III) ions. + Construct the equation for the reaction between iron(II) ions, hydrogen ions, H , and oxygen to form iron(III) ions and water. Solution 1ai. In cold water. Products: sodium hydroxide and hydrogen 1aii. Heated with steam. Products: magnesium oxide and hydrogen 1bi. filtration: solid particles are removed. 1bii. chlorination: germs and bacteria are killed by sterilising water with chlorine. 1c. 4Fe 2+ + 3+ (aq) + O2 (g) + 4H (aq) ---> 4Fe (aq) + 2H2O (l) 2. Calcium oxide is produced by heating a mixture of limestone and coke in a lime kiln. CaCO3 <---> CaO + CO2 ai. Explain the meaning of the symbol <---> aii. In the lime kiln, the carbon dioxide is allowed to escape. Why does this increase the yield of calcium oxide? b. The calcium oxide reacts with water to form slaked lime. i. Give the equation for this reaction ii. State a use of slaked lime Solution 2ai. It shows that the decomposition of calcium oxide in the lime kiln is a reversible reaction. 2aii. The decrease in carbon dioxide concentration causes the equilibrium to shift to the right to produce more carbon dioxide to replace those that escaped. Therefore, more calcium carbonate decomposes to give calcium oxide. 2bi. CaO + H2O --> Ca(OH)2 2bii. It is used to treat acidic soils. It reacts with acid to produce salt and water. 3. Choose from the following metals to answer the questions below. aluminium calcium copper iron magnesium potassium sodium zinc Each metal can be used once, more than once, or not at all. Name a metal which a. is manufactured by the electrolysis of its molten oxide b. has a variable valency c. is used to galvanise iron d. has a carbonate which is coloured e. is alloyed with zinc to make brass Solution 3a. aluminim 3b. copper/iron 3c. zinc 3d. copper 3e. copper 4. In separate experiments, powdered samples of metal X and metal Y reacted with solutions of nickel(II) sulphate and of iron(II) sulphate. The following table shows how the colours of the solutions changed. metal X metal Y nickel(II) sulphate Solution goes from green to colourless Solution goes from green to colourless iron(II) sukphate Solution stays pale green Solution goes from pale green to colourless a. predict the order of reactivity for the four metals X, Y, nickel, and iron. b. Metal Y was placed in aqueous copper(II) sulphate. i. What colour change was seen? ii. Give one other observation c. Write the ionic equation, with state symbols, for the reaction between iron and aqueous nickel(II) sulphate. Solution a. from least reactive to most reactive: nickel, metal X, iron, metal Y bi. blue copper(II) sulphate solution decolourises bii. a reddish brown deposit is formed 2+ c. Fe (s) + Ni (aq) ----> Fe 2+ (aq) + Ni (s