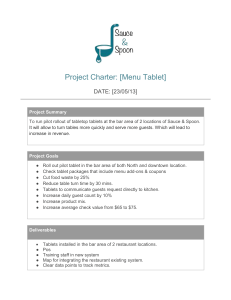
Industrial Internship Exit Exam 2022 1. Residual method titration with EDTA is applicable to what metal ion:* 1 point A) Calcium B) Aluminum C) Zinc D) Magnesium 2. Cross contamination can come from the following, EXCEPT:* 1 point a. Unclean clothing b. Airborne particles c. Improper dispensing d. Inaccuracies in mix-ups 3. The number of mg of KOH needed to neutralize the free acids and saponify the esters in 1g of oil or fat:* 1 point A) Acid value B) Ester value C) Saponification value D) Iodine value 4. The masking agent used in the assay of Mg with EDTA in the presence of Al is:* 1 point A) Triethanolamine B) Thioglycol C) Potassium cyanide D) Ammonium fluoride 5. The following statement/s is/are true regarding gels: I. Gels may thicken on standing, forming a thixotrope and must be shaken before use. II. Milk of magnesia is an example of a single-phase gel III. Gels and jellies are two different terms* 1 point A. I only B. I & II C. II only D. I & III E. I, II, III 6. The following can be formulated as TDDS Scopolamine II. Nicotine III. I. Clonidine* 1 point A. I only B. III only C. I&III D. II&III E. I, II, III 7. The following statement/s is/are true for pastes: contain a smaller proportion of solid material than I. They generally ointments. II. They are less greasy and less stiffer than their counterpart ointments due to reduced amount of based used. III. They remain in place after application and are effectively employed to absorb serous secretions.* 1 point A. I only B. I&II C. I&III D. III only E. I, II, III 8. The method of mixing or blending small amounts of powders by movement of spatula through them on a sheet of paper or ointment slab is called* 1 point a. Levigation b. Comminution c. Spatulation d. Trituration 9. Transdermal Drug Delivery System: I. Avoids gastrointestinal drug absorption difficulties II. Avoids the occurrence of contact dermatitis III. Drug therapy cannot be terminated rapidly.* 1 point A. I only B. III only C. I&III D. II&III E. I, II, III 10. Constant weight in analytical procedures of drying means that consecutive weighing after heating and cooling do not differ by* 1 point A) More than 0.25mg B) More than 0.50mg C) More than 0.255mg D) Not more than 0.75mg 11. An example of primary packaging material is a* 1 point a. Insert b. Brochure c. Label d. Seal 12. Nephelometry is based on the measurement of light that is:* 1 point A) Refelected B) Absorbed C) Transmitted D) Adsorbed by the particles of a suspension 13. Oils with iodine value above 120 are classified as* 1 point A) Non-drying B) Semi-drying C) Drying D) None of the above 14. Film coated tablets possess the following characteristic/s: destruction by abrasion than are sugar coated tablets colored to make tablets attractive and distinctive may be non-aqueous or aqueous* I. Less resistant to II. Coating may be III. Film-coating solutions 1 point A. I only B. I & II C. II only D. II & III E. III only 15. This problem corresponds to the filling-in of the score line or indented logo on the tablet by the film.* 1 point A. peeling B. picking C.orange-peel effect D. mottling E. bridging 16. The functions of Quality Control include the following, except:* 1 point A) Analytical control B) Inspection control C) Auditing D) A and B 17. Solid preparations made up of sodium bicarbonate, citric acid, and tartaric acid are referred to as* 1 point a. Effervescent granules b. Bulk powder c. Divided powder d. Aerosol granules 18. Retention period for finished products should be* 1 point a. At least 2 years after the distribution of the first lot b. One year after the expiration date of the product c. At least 3 years after the manufacture is completed d. At least 1 year after the expiration date of the last lot 19. Hard gelatin capsules contain ______% of water* 1 point a. 12-16 b. 10-20 c. 0.1-1 d. 1-10 20. The following statement/s is/are true for wet granulation method: I. Liquid binder is added to the powder mixture to facilitate the adhesion of the powder particles II. Over-wetting of the powder can result in granules that are too soft for proper tableting and under-wetting can result in tablets that are too hard III. Granules may be dried in thermostatically controlled ovens which constantly record the time, temperature and humidity.* 1 point A. I only B. I & II C. I & III D. II & III E. I, II, III 21. The main concerns for the formulation of suspensions are the following, EXCEPT:* 1 point a. To eliminate separation on standing b. To create the required particle size c. To decrease rate of settling d. To permit easy resuspendability 22. In the Statistical Quality Control, the chart used to measure the variations in the products inspected in the production:* 1 point A) Variable chart B) P-chart C) Attribute chart D) B and C 23. A plot of absorbance against concentration of a standard drawn in straight line is:* 1 point A) Charle’s B) Beer’s C) Lambert’s D) B and C 24. The design objectives of TDDS include: I. To deliver the drug at an optimal rate of the skin for percutaneous absorption at the therapeutic levels II. To adhere well to the patient’s skin and have a patch-size, appearance and site-placement that encourage patient acceptance III. To occlude the skin to ensure the one-way flux of the drug into the stratum corneum* 1 point A. I only B. III only C. I&III D. II&III E. I, II, III 25. Small, usually cylindrical, molded or compressed tablets containing small amounts of usually potent drugs are known as* 1 point a. Dispensing tablets b. Chewable tablets c. Hypodermic tablets d. Tablet triturates 26. Trace minerals in a multivitamin preparation can be assayed faster by:* 1 point A) Atomic Absorption Spectrophotometer B) UV-Vis Spectrophotometer C) Gas Liquid Chromatography D) High Pressure Liquid Chromatography 27. The gram equivalent of Magnesium oxide (MW = 40.30) is* 1 point A) 0.0403 B) 0.0215 C) 21.15 D) 0.4030 28. Aspirin, which is hydrolyzed on exposure to moisture, is prepared into tablet using the dry granulation method. Other drugs which should be prepared using this process include: I. Ascorbic acid II. Methenamine III. Thiamine HCl* 1 point A. I only B. I & II C. I & III D. II & III E. I, II, III 29. Tablets that are prepared by subjection to more than a single compression are called* 1 point a. Enteric-coated tablets b. Film-coated tablets c. Multiple compressed tablets d. Molded tablets 30. Which of the following is NOT used as a diluent or absorbent in the formulation of capsules?* 1 point a. Lactose b. Magnesium carbonate c. Kaolin d. Light magnesium Oxide e. Starch 31. This is a problem often encountered in film coating process characterized by roughness of the tablet surface due to failure of spray droplets to coalesce* 1 point A. peeling B. picking C.orange-peel effect D. mottling E. bridging 32. Pessaries: I. cocoa butter is the base Usually globular or oviform and weighs 2g each when II. Employed principally to combat infections occurring in the female genitourinary area III. The most commonly used base consist of combination of the various molecular weight PEG* 1 point A. I only B. III only C. I&III D. II&III E. I, II, III 33. A process wherein the sample is made to liberate iodine, which is then titrated with a standard solution of sodium thiosulfate* 1 point A) Volumetric titration B) Back titration C) Iodometry D) Iodimetry 34. Type of alkaloidal assay where the total alkaloids are determined:* 1 point A) Ultimate B) Specific C) Extraction D) Proximate 35. The process of removing an appropriate number of items from a population in order to make interferences to the entire population is called:* 1 point A) Sampling B) Inspection C) Statistic D) None of the above 36. Inspection stations are placed in the following areas, except:* 1 point A) Analytical laboratory B) Manufacturing area C) Warehouse D) Packaging area 37.The iodine value of oils is a quantitative measure of:* 1 point A) Phenol content B) Saturated fatty acids C) Unsaponifiable matter D) Unsaturated fatty acids 38.Which of the following is frequently used as a peel strip for transdermal patches?* 1 point a. Polyesters b. Acrylic c. Silicone d. Polyisobutylene e. Starch 39.HEPA filters are used for the filtration of* 1 point a. Parenterals b. Ophthalmics c. Air d. Solvents 40. A buret with a glass stopcock can be used for:* 1 point A) Alcohols B) Acids C) Bases D) Salts 41. Which of the following substances is assayed by residual alkalimetric analysis?* 1 point A) Aspirin B) Sodium hydroxide C) Hydrochloric acid D) Zinc oxide 42. The type of suspension system in which the particles settles as a dense sediment is called* 1 point a. Flocculated system b. Colloidal system c. Coagulated system d. Deflocculated system 43. Particle size can be influenced by the following factors, EXCEPT:* 1 point a. Penetrability of particles intended to be inhaled for deposition deep in the respiratory tract b. Grittiness of solid particles c. Uniform distribution of a drug substance d. Suspendability of particles intended to remain undissolved but uniformly dispersed in liquid vehicle 44. The study of the optical activity of a substance is:* 1 point A) Polarimetry B) Refractometry C) Spectrophotometry D) Potentiometry 45. The selection of a permeation enhancer in developing a TDDS should be based on: I. Efficacy in enhancing skin permeation II. Biocompatibility with other components components* III. Physicochemical compatibility with other 1 point A. I only B. III only C. I&III D. II&III E. I, II, III 46. This method is used when a small amount of potent substances is to be mixed with a large amount of diluents* 1 point A. Block and divide method B. Spatulation C. geometric dilution D. sifting E. trituration 47. Petrolatum, USP is:* 1 point A. A purified mixture of semi-solid hydrocarbons from petroleum that has been wholly or nearly decolorized B. Also known as Yellow ointment C. Is also known as white Vaseline D. Water-soluble E. Water-washable 48. Which of the following powders can be classified as bulk powders? I.Douche III. Insufflation* II. Dusting powder 1 point A. I only C. I and II B. III only D. II and III E. I, II and III 49. The indicator for EDTA direct titration of calcium carbonate:* 1 point A) Thymol blue B) Hydroxynaphthol blue C) Methyl red D) Methylene blue 50. Which of the following is NOT used as a diluent in the formulation of a tablet?* 1 point a. Celutab b. Corn starch c. Lactose d. Avicel e. Ethyl cellulose 51. Process employed both to comminute and mix powders is called* 1 point a. Trituration b. Blending c. Spatulation d. Levigation 52. Analysis wherein the constituents of a sample are separated and then the product is weighed:* 1 point A) Volumetric B) Gravimetric C) Special method D) Gasometric 53. It is used for compacting the materials in the tablet machine.* 1 point a. Hopper b. Feed frame c. Dies d. Punches 54. In paper chromatography, the data needed to compute for Rf value:* 1 point A) Distance traveled by the solute B) Distance traveled by the solvent C) A and B D) Distance traveled by the blank 55. The required amount of methyl and propyl paraben combinations as preservatives employed in pharmaceutical products is* 1 point a. 0.1-0.2% b. 0.1-0.5% c. 15-20% d. 0.002-0.01% 56. A functional requirement for Rubber Closures is* 1 point a. It should be easily subjected to sterilization. b. It should not provide protection against bacterial contamination. c. It should not make bacteria-proof seals. d. It should provide complete barrier against vapor transport. 57. The information that gives an indication of particle size and size range of the raw material along with the crystal structure is the* 1 point a. Microscopic examination b. Physical description c. Particle size d. Solubility 58. The measurement of a base of a given sample by titration with a standard acid is:* 1 point A) Acidimetry B) Compleximetry C) Alkalimetry D) Redox 59. The hydrocarbon base which is frequently used as a vehicle for ointment.* 1 point a. Cholesterol b. Lanolin c. Petroleum d. Sorbitan monostearate monooleate e. Mineral oil 60. The Kjeldahl method of analysis is used to determine:* 1 point A) Fats B) Sugars C) Nitrogen D) Oxygen in organic compounds 61. The following statement/s is/are true about percutaneous absorption: I. Drugs penetrate through the skin better in their unionized form. II. Non-polar drugs tend to cross the cell barrier through the lipid-rich regions (transcellular route) whereas the polar drugs favor transport between cells (intracellular) III. More drugs are absorbed when the drug substance is applied and concentrated on a smaller surface area.* 1 point A. I only B. III only C. I&III D. II&III E. I, II, III 62. A sample of 0.1350g of arsenic trioxide (As2O3) was assayed iodimetrically using 23.4mL of 0.1055N iodine solution. The percentage purity of the sample is:* 1 point A) 90.44 B) 89.10 C) 90.54 D) 90.23 63. This is the primary cause of product instability and involves the addition of oxygen or the removal of hydrogen:* 1 point A) Incompatibility B) Oxidation-Reducation C) Hydrolysis D) Racemization E) Decarboxylation 64. Powders containing deliquescent and hygroscopic materials should be wrapped in what kind of paper? I. Vegetable parchment II. Glassine paper III. Waxed paper* 1 point A. I only B. III only C. I and II D. II and III E. I,II and III 65. A tool for detecting variations in a process:* 1 point A) Pie chart B) Bar chart C) Quality control chart D) T-chart 66. The following are methods of sterilization for parenteral products, EXCEPT:* 1 point a. Steam sterilization b. Boiling c. Filtration d. Gas sterilization 67. A sample of Magnesia Magma weighing 12.35g was dissolved in 50mL of 1.0340N sulfuric acid and titrated until end point is reached consuming 24.6mL of 1.1255N sodium hydroxide solution. The percentage of MgO content is: At.Wt. of Mg = 24.3: O = 16* 1 point A) 3.92 B) 4.0 C) 3.85 D) 3.91 68. These tablets are prepared so that an initial dose of drug is released immediately followed later by a second dose.* 1 point A. Extended-release B. Delayed-release C. Repeat action D. Modified-release E. Targeted release 69. The molecular weight of NaOH is 40. How many grams of NaOH pellets are needed to make 500mL of 1.5N solution?* 1 point A) 20 B) 30 C) 40 D) 60 70. Using a sampling plan by military standard 105 D, the sample size required is obtained from* 1 point A) Master table B) Ratio and proportion C) Samples needed D) A and B 71. Materials to be sampled include the following, except:* 1 point A) Final products B) Records C) Intermediate products D) Raw materials 72. The Quality Control Department is important to:* 1 point A) Ensure uniform production of high quality product B) Safeguard public health C) Ensure that minimum standards of the product comply with the BFAD requirements D) All of the above 73. An accurately measured sample of hydrogen peroxide 2g was dissolved in a mixture of 20mL water and 20mL diluted sulfuric acid and was titrated with 0.1N potassium permanganate consuming 30mL to reach the endpoint. Compute for the percentage of hydrogen peroxide (MW=34):* 1 point A) 2.55% B) 5.1% C) 2.5% D) 5.5% 74. This type of dosage form allows a reduction in dosing frequency to that presented by a conventional dosage form.* 1 point A. Extended-release B. Delayed-release C. Repeat action D. Modified-release E. Targeted release 75. The study of the deformation and flow of liquid and semi-solid preparations referred to as* 1 point a. Emulsification b. Suspendability c. Compatibility study d. Rheology 76. A special quality control test done for suspensions to measure the degree of air entrapped is called* 1 point a. Specific gravity b. Coulter counter c. Photomicroscopy d. Physical stability 77. This problem is characterized by the appearance of small amounts of film fragments flaking from the tablet surface.* 1 point A. peeling B. picking C. orange-peel effect D. mottling E. bridging 78. The following compounds are assayed by acidimetrically, except:* 1 point A) Sodium hydroxide B) Caffeine C) Citric acid D) Zinc oxide 79. Types of liquids that may be encapsulated into soft gelatin capsules include the following: I. Vegetable and aromatic oils glycol II. Propylene III. Polyethylene glycols* 1 point A. I only B. III only C. I and II D. II and III E. I, II, and III 80. The main methods used in the preparation of purified water are distillation and ion-exchange. In distillation: I. The first 5% of the aqueous distillate must be discarded II. The last portion of the water about 100% of original volume, remaining in the distillation apparatus must be discarded III. Water purified in this manner is referred to as demineralized water* 1 point A. I only B. II only C. I & III D. II & III E. I, II, III 81. The factor/s which play/s a part in percutaneous absorption is/are: Molecular weight solubility* II. 1 point A.I only B. II only C. I&II D. II&III E. I, II, III 82. The moisture content of a drug may be:* Partitioning coefficient I. III. 1 point A) Water of hydration B) Water in the absorbed form C) Water of emulsion D) A and B 83. This determines the shelf life of a product:* 1 point A) Sampling inspection program B) All of the answers C) Validation program D) Stability testing program 84. Most of the official drugs containing calcium and zinc are assayed by:* 1 point A) EDTA method B) Gravimetry C) Nonaqueous titrimetry D) Acidimetry 85. The following statement/s is/are true about percutaneous absorption: I. The amount of drug percutaneously absorbed per unit of surface area per time interval increase as the concentration of the drug substance in the transdermal drug delivery system is increased. II. The hydration of the skin hinders percutaneous absorption III. The longer the period of time the medicated application is permitted to remain in contact with the skin, the greater will be the total drug absorption.* 1 point A. I only B. III only C. I&III D. II&III E. I, II, III 86. The First-In First-Out Policy must always be observed to:* 1 point A) Assures that the oldest stock is used first B) Prevents contamination and mix-ups of materials C) Contains the information regarding the activity of the active ingredient D) All of the above 87. This type dosage form is designed to release the drug form at a time other than promptly after administration.* 1 point A. Extended-release B. Delayed-release C. Repeat action D. Modified-release E. Targeted release 88. The period of stability of a preparation is the time from the date of manufacture until its chemical or biological activity* 1 point a. Has deteriorated b. Is close to 100% c. Is not less than 90% of the labeled potency d. Has reached its maximum 89. _________ are substances that make up the major portion of tablet.* 1 point a. Binder b. Glidant c. Lubricant d. Diluent e. Disintegrant 90. It is the process of comminution in which a paste is formed by combining the powder material and a small amount of liquid in which the powder is insoluble. I. levigation II. Pulverization by intervention III.Spatulation* 1 point A. I only B. III only C. I and II D. II and III E. I, II and III 91. This substance provides water solubility or permeability to the film to ensure penetration by body fluids and therapeutic availability of the drug.* 1 point A. alloying substance B. Plasticizer C. film former D. surfactant E. glossant 92. A common sampling plan that uses master tables to interpret the results:* 1 point A) 100% inspection B) Square root system C) Military standard D) A and C 93. The indicator used in permanganate titration* 1 point A) Methyl orange B) Phenolphthalein C) Permanganate solution D) Methyl red 94. The concentration of an unknown sample in spectrophotometric procedures can be calculated by* 1 point A) Use of Beer’s plot B) Use of chemical factor C) Use of mathematical formula using a reference standard data D) A or C 95. A major process by which foreign substances, including drugs, are eliminated from the body is called* 1 point a. Metabolism b. Distribution c. Absorption d. Liberation 96. Used for mixing powder mixtures in large volume quantity* 1 point a. Drum roller b. V-blender c. Planetary mixer d. Compactor mills 97. In the assay of acetic acid (MW = 60.05), each mL of 1N NaOH is equivalent to:* 1 point A) 0.60000g of acetic acid B) 0.00605g of acetic acid C) 0.60050g of acetic acid D) 0.06005g of acetic acid 98. The ash content of an organic compound is an impurity of:* 1 point A) Carbon B) Oil C) Inorganic matter D) Volatile component 99. The IPQC Test that determines the durability of a tablet and its ability to withstand abrasion in handling, packaging and shipment; USP requires a maximum weight loss of not more than 1% is the* 1 point a. Tablet hardness b. Tablet disintegration c. Tablet thickness d. Friability test 100. In preparing effervescent granulated salts, which of the following statement/s hold/s true? I. Effervescent granules can be prepared using two methods, the dry and wet methods. II. The effervesce from the released CO2 serves to mask the bitter or salty taste of drugs. III. Using tartaric acid as the sole acid would result in a sticky mixture which is difficult to granulate.* 1 point A. I only B. III only C. I and II D. II and III E. I, II and III 101. Limulus ameobocyte lysate (LAL) assay method is applicable in the determination of:* 1 point A) Pyrogens B) Chemical impurities C) Microorganisms D) All of the above 102. The sweetening agent employed to impart sweetness to chewable tablets is* 1 point a. Dextrose b. Saccharin Sodium c. Mannitol d. Sucrose