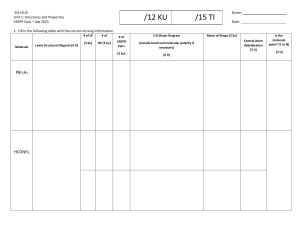
POLARITY OF MOLECULES AND MOLECULAR GEOMETRY POLARITY OF A MOLECULE • IT REFERS TO HOW EVENLY THE ELECTRONS ARE SHARED BETWEEN THE ATOMS THAT MAKE UP THE MOLECULE. • ELECTRONS ARE SHARED EVENLY = THE MOLECULE IS NONPOLAR • ELECTRONS ARE NOT SHARED EVENLY = THE MOLECULE IS POLAR MOLECULAR GEOMETRY • THE SHAPE OF A MOLECULE DEPENDS ON THE ARRANGEMENT OF ITS ATOMS, AND THIS ARRANGEMENT AFFECTS THE DISTRIBUTION OF ELECTRONS IN THE MOLECULE. TWO KEY FACTORS THAT DETERMINE MOLECULAR GEOMETRY: 1. THE NUMBER OF ELECTRON PAIRS AROUND THE CENTRAL ATOM 2. THE NUMBER OF ATOMS BONDED TO THE CENTRAL ATOM BASED ON THESE FACTORS, WE CAN PREDICT THE SHAPE OF A MOLECULE USING A MODEL CALLED THE VALENCE SHELL ELECTRON PAIR REPULSION (VSEPR) THEORY. VALENCE SHELL ELECTRON PAIR REPULSION (VSEPR) THEORY • THE VSEPR THEORY STATES THAT ELECTRON PAIRS AROUND THE CENTRAL ATOM WILL REPEL EACH OTHER AND SPREAD OUT AS FAR AS POSSIBLE, LEADING TO SPECIFIC GEOMETRIC SHAPES. • THE SHAPE OF A MOLECULE IS CRUCIAL IN DETERMINING ITS POLARITY. • IN A NONPOLAR MOLECULE, THE ELECTRONS ARE SHARED EVENLY BETWEEN THE ATOMS, AND THE MOLECULE HAS A SYMMETRICAL SHAPE. • IN CONTRAST, IN A POLAR MOLECULE, THE ELECTRONS ARE NOT SHARED EVENLY, AND THE MOLECULE HAS AN ASYMMETRIC SHAPE.