![Co(III) Complex Synthesis: [Co(NH3)5Cl]Cl2 Lab Experiment](http://s2.studylib.net/store/data/026168368_1-8dd222755818d8e28a307a668b492ff8-768x994.png)
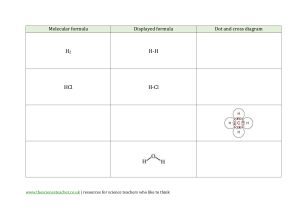
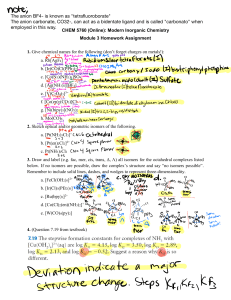
Experiment 3 – Part B Part B: Synthesis of [Co(NH3)5Cl]Cl2 Objective: To prepare and isolate a Co(III)-based coordination compound starting from the product of Experiment 4a. The solid is also to be used in additional experiments involving conductivity, UVVis, and infrared spectroscopy. Pre-Lab Study: You should include equations (3), (4), and (5) of the introduction in your Pre-lab for Exp. 4b. Also, remember to save some of you intermediate product for conductivity, UV-vis and IR studies to be performed in Exp. 4c & 4d. Background: Of particular importance to the development of coordination chemistry are metal complexes of the type to be synthesized and characterized in this experiment. Prior to 1950, research in this area was almost exclusively concerned with the investigation of complexes of transition metal ions with such monodentate ligands as Cl-, Br-, I-, NH3, pyridine, CN-, and NO2and bidentate ligands as ethylenediamine (H2NCH2CH2NH2), oxalate (-O2CCO2-), glycinate (H2NCH2CO2-), and CO32-. These complexes form the basis of a vast amount of research today, despite the more recent discoveries of the ligand properties of H, CH 3, CO, H2C=CH2, and benzene, to mention a few. Coordination compounds of Co(III) and Cr(III) have been of particular interest because their complexes undergo ligand exchange very slowly compared with complexes of many other transition metal ions. For example, Ni(NH3)62+ reacts instantaneously with H2O to form Ni(OH2)62+. Under the same conditions, the analogous reactions of Co(NH3)63+ and Cr(NH3)63+ occur very slowly. This difference in behavior of complexes of different metal ions has been qualitatively accounted for by ligand field theory and molecular orbital theory. The slow reactivity of Co(III) complexes has made them suitable for extensive investigations. The structures of the octahedral Co(III) complexes that you will prepare are given below. H3N NH3 H3N O Co NH3 NH3 O NO3 O NH3 NH3 NH3 n Cl2 Co Cl NH3 n One important method of characterizing ionic substances is the determination of the ability of their solutions to conduct an electric current. Those substances whose solutions have the highest conductivity consist of the greatest number of ions. Thus, a one molar solution of [Co(NH3)4CO3]NO3 will have a lower conductance than a solution of [Co(NH3)5Cl]Cl2 of the same concentration. By measuring the conductivity of a solution of a compound, it is possible to determine whether a formula unit of that compound consists of 2, 3, 4 or more ions. Although measurements will be done on water solutions of the complexes, the same information can be frequently obtained using organic solvents such as nitrobenzene or acetonitrile for ionic compounds that are either not very soluble in water or react with water. Another very powerful method for establishing the identity of a complex is infrared spectroscopy. This technique examines the frequencies of the vibrational modes of a molecule. Thus, the infrared spectra of both of the preceding complexes exhibit absorptions at frequencies (commonly expressed in wave numbers, cm-1, i.e. reciprocal wavelength 1/) characteristic of stretching and bending modes of the NH3 group. While the Co-N stretching modes are in principle also measurable, they sometimes occur at frequencies too low (below 600 cm-1) to be observed in most infrared spectrophotometers. More specialized and expensive infrared instruments are, however, available for studying these vibrations. The complex [Co(NH3)4CO3]NO3 also exhibits absorptions characteristic of the carbonate group. Because of its coordination to the metal ion, the CO32- group has a somewhat different spectrum in this complex than it has as the ion, as in Na2CO3. The spectrum should also contain absorptions bands resulting from vibrational modes of the NO3ion, very similar to those observed in NaNO3. In contrast the spectrum of [Co(NH3)5Cl]Cl2 would be largely dominated by absorptions attributable to the NH3 groups. In general, metal-Cl stretching frequencies are lower than can be observed on usual infrared instruments, and the ionic Cl- groups, of course, are not, in the solid state, strongly bonded to any other single atoms; thus, no absorptions are expected in the infrared spectrum to indicate their presence in the compound. While infrared spectroscopy and other instrumental methods of compound characterization are emphasized in this and in other experiments in this book, it should be stressed that a quantitative elemental analysis is an absolutely essential step in determining the composition and structure of a new compound. The synthesis of [Co(NH3)4CO3]NO3 will be carried out according to the unbalanced equation, Co(NO3)2 + NH3 (aq) + (NH4)2CO3 + H2O2 [Co(NH3)4CO3]NO3 + NH4NO3 + H2O (1) The Co(NO3)2 that is available commercially has the formula Co(NO3)2 • 6 H2O and very probably is a coordination compound having the ionic formulation [Co(OH2)6](NO3)2. Since Co(II) complexes, like those of Ni(II), react very rapidly by ligand exchange, the first step in the reaction might be expected to be, Co(OH2)62+ + 4 NH3 + CO32- Co(NH3)4CO3 + 6 H2O (2) This Co(II) complex could then be oxidized by the transfer of an electron to H2O2 to give the relatively non-reactive Co(III) ion, [Co(NH3)4CO3]+. The preparation of [Co(NH3)5Cl]2+ is accomplished from the carbonato complex according to the following series of equations: [Co(NH3)4CO3]+ + 2 HCl [Co(NH3)4(OH2)Cl]2+ + CO2 (g) + Cl- (3) [Co(NH3)4(OH2)Cl]2+ + NH3 (aq) [Co(NH3)5(OH2)]3+ + Cl- (4) [Co(NH3)5(OH2)]3+ + 3HCl [Co(NH3)5Cl]Cl2 (s) + H2O + 3H+ (5) On the basis of mechanistic studies of reactions of [Co(NH3)4CO3]+ with acids, the first reaction in the preceding sequence probably involves the following mechanism: + O (NH3)4 Co 2+ + Cl 2 H (NH3)4 Co O O Cl Cl + (NH3)4 Co O n O OH2 CO2 n O Chemicals Used: Product from Exp. 3a – [Co(NH3)4CO3]NO3 Concentrated Hydrochloric acid – HCl Ammonium Hydroxide – NH4OH Procedure: You are encouraged to make observations of color changes and precipitate formations during the course of each part of this experiment. Chloropentaamminecobalt(III) Chloride, [Co(NH3)5Cl]Cl2: dissolve 3.0 g of [Co(NH3)4CO3]NO3 in 30 mL of H2O and add 3 to 6 mL of concentrated HCl until all of the CO2 is expelled. The 3-6 mL of the concentrated, 12 Molar HCl(aq) can be added slowly until no further gas is evolved and all of the intermediate has gone into the solution. Record the amount of acid utilized. Neutralize with concentrated aqueous NH3 and then add about 3 mL excess. It is important that any excess acid has been neutralized and then that the required excess of the 15 Molar, ammonia has been added. If you cannot be sure whether the solution has been neutralized by directly testing of the liquid with full-range litmus paper, hold a moistened litmus above the solution in the beaker. Once the first sign that the gas over the solution is basic, you can assume neutralization has been achieved. Then heat the solution to between 80-90 C on a hot plate for 15 minutes until the [Co(NH3)5H2O]3+ complex has formed. Avoid boiling. Note the solution color. Sometimes a slight precipitate of the hydroxo-complex is observed at this point. Cool the solution to below 40 C in an ice bath and then add the 50 mL of conc. HCl. Note the color of the precipitate that should form at this point. Reheat the solution to between 75-85°C for 15-20 minutes and observe the color changes. Wash the compound with small amount (1-2 mL) of ice-cold distilled water; then filter under a water aspirator vacuum with a glass fritted funnel. The final solid present should be purple in color. Calculate the percent yield. (Do not discard the compound because part of it will be needed in Experiment 4c & 4d). Waste: Cobalt filtrate from the synthesis should be deposited in the appropriately labeled waste container. Post Lab Report Experiment 4: Part B (100 points) 1. (20 points) Describe the amount (g, moles) of the carbonato complex and the volume and moles of HCl that were used in the first step of the reaction and any observations. Give a balanced chemical equation for the reaction occurring at this step. Name the new complex ion formed (consistent with IUPAC standards) and sketch its structure. There are two geometrical isomers possible for this complex, include both in your sketch. Which one do you think is more likely to be present in this case? Why? 2. (20 points) Describe the next step in the procedure that the above solution was carried through. Give a balanced chemical equation for the reaction you are conducting at this point using the excess ammonia. Name the new complex formed in the solution at this point, sketch its structure and indicate its overall charge. 3. (20 points) In the next step excess ammonia was neutralized with HCl and excess HCl added. State the amount of the acid you used and any observations made. Many students noted the appearance of a salmon (orange-red) precipitate at this point. This precipitate is a chloride salt of the complex formed in the preceding step whose solubility is reduced through the common-ion effect. Write a net ionic equation for this precipitation and explain the common-ion effect on reducing solubility of a salt. 4. (20 points) Describe your observations with regard to subsequent heating of the mixture in HCl and give a balanced chemical equation for the formation of the desired final chloropentaammine cobalt salt. Describe how the final compound was isolated, washed and dried and state the final amount obtained along with any distinctive physical characteristics of the compound. 5. (20 points) Yield Calculation. Give a balanced, overall molecular equation for the synthesis of the final chloropentaamminechloride salt from the carbonatotetraammine nitrate. What evidence did you obtain for possible side reactions in production of the final product that would account for some lowering of the yield?