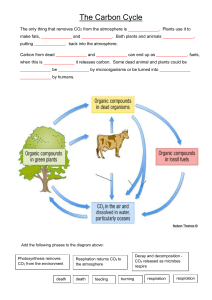
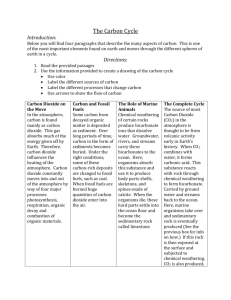
The Carbon Cycle and Earth's Climate Carbon dioxide is an atmospheric constituent that plays several vital roles in the environment. It is a greenhouse gas that traps infrared radiation heat in the atmosphere. It plays a crucial role in the weathering of rocks. It is the carbon source for plants. It is stored in biomass, organic matter in sediments, and in carbonate rocks like limestone. The Carbon Cycle The primary source of carbon/CO2 is outgassing from the Earth's interior at midocean ridges, hotspot volcanoes, and subduction-related volcanic arcs. Much of the CO2 released at subduction zones is derived from the metamorphism of carbonate rocks subducting with the ocean crust. Much of the overall outgassing CO2, expecially as midocean ridges and hotpot volcanoes, was stored in the mantle when the Earth formed. Some of the outgassed carbon remains as CO2 in the atmosphere, some is dissolved in the oceans, some carbon is held as biomass in living or dead and decaying organisms, and some is bound in carbonate rocks. Carbon is removed into long term storage by burial of sedimentary strata, especially coal and black shales that store organic carbon from undecayed biomass and carbonate rocks like limestone (calcium carbonate). Photosynthesis Plants and photosynthetic algae and bacteria use energy from sunlight to combine carbon dioxide (C02) from the atmosphere with water (H2O) to form carbohydrates. These carbohydrates store energy. Oxygen (O2) is a byproduct that is released into the atmosphere. This process is known as photosynthesis. carbon dioxide + water + sunlight -> carbohydrate + oxygen CO2 + H2O + sunlight -> CH2O + O2 Respiration Plants (and photosynthetic algae and bacteria) then use some of the stored carbohydrates as an energy source to carry out their life functions. Some of the carbohydrates remain as biomass (the bulk of the plant, etc.). Consumers such as animals, fungi, and bacteria get their energy from this excess biomass either while living or dead and decaying. Oxygen from the atmosphere is combined with carbohydrates to liberate the stored energy. Water and carbon dioxide are byproducts. oxygen + carbohydrate -> energy + water + carbohydrate O2 + CH2O -> energy + H2O + CO2 Notice that photosynthesis and respiration are essentially the opposite of one another. Photosynthesis removes CO2 from the atmosphere and replaces it with O2. Respiration takes O2 from the atmosphere and replaces it with CO2. However, these processes are not in balance. Not all organic matter is oxidized. Some is buried in sedimentary rocks. The result is that over geologic time, there has been more oxygen put into the atmosphere and carbon dioxide removed by photosynthesis than the reverse. Weathering Carbon dioxide and the other atmospheric gases dissolve in surface waters. Dissolved gases are in equilibrium with the gas in the atmosphere. Carbon dioxide reacts with water in solution to form the weak acid, carbonic acid. Carbonic acid disassociates into hydrogen ions and bicarbonate ions. The hydrogen ions and water react with most common minerals (silicates and carbonates) altering the minerals. The products of weathering are predominantly clays (a group of silicate minerals) and soluble ions such as calcium, iron, sodium, and potassium. Bicarbonate ions also remain in solution; a remnant of the carbonic acid that was used to weather the rocks. Carbonate Rocks 1. Carbon dioxide is removed from the atmosphere by dissolving in water and forming carbonic acid CO2 + H2O -> H2CO3 (carbonic acid) 2. Carbonic acid is used to weather rocks, yielding bicarbonate ions, other ions, and clays H2CO3 + H2O + silicate minerals -> HCO3- + cations (Ca++, Fe++, Na+, etc.) + clays 3. Calcium carbonate is precipitated from calcium and bicarbonate ions in seawater by marine organisms like coral Ca++ + 2HCO3- -> CaCO3 + CO2 + H2O the carbon is now stored on the seafloor in layers of limestone Metamorphism of Carbonates Some of this carbon is returned to the atmosphere via metamorphism of limestone at depth in subduction zones or in orogenic belts CaCO3 + SiO2 -> CO2 + CaSiO3 followed by outgassing at the volcanic arc. The Greenhouse Effect Most of the sun's energy that falls on the Earth's surface is in the visible light portion of the electromagnetic spectrum. This is in large part because the Earth's atmosphere is transparent to these wavelengths (we all know that with a functioning ozone layer, the higher frequencies like ultraviolet are mostly screened out). Part of the sunlight is reflected back into space, depending on the albedo or reflectivity of the surface. Part of the sunlight is changed into infrared (lower frequency than visible light). While the dominant gases of the atmosphere (nitrogen and oxygen) are transparent to infrared, the so-called greenhouse gasses, primarily water vapor (H2O), carbon dioxide, and methane (CH4), absorb the infrared radiation. They collect this heat energy and hold it in the atmosphere. While we worry about possible global warming from the additional CO2 we put into the atmosphere by burning fossil fuels, if there was no CO2 in the atmosphere the global climate would be significantly cooler. The Climate Buffer Because of the role of CO2 in climate, feedbacks in the carbon cycle act to maintain global temperatures within certain bounds so that the climate never gets too hot or too cold to support life on Earth. The process is a large-scale example of LeChatelier's Principle. This chemical principle states that if a reaction at equilibrium is perturbed by the addition or removal of a product or reactant, the reaction will adjust so as to attempt to bring that chemical species back to its original concentration. For example, as carbonic acid is removed from solution by weathering of rocks, the reaction will adjust by producing more carbonic acid. And since the dissolved CO2 is in equilibrium with atmospheric CO2, more CO2 is removed from the atmosphere to replace that removed from solution by weathering. some examples: If CO2 concentration increases in the atmosphere because of an increased rate of outgassing, global temperature will rise. Rising temperature and more dissolved CO2 will lead to increased weathering of crustal rocks as a result of faster reaction rates (temperature effect) and greater acidity. Enhanced weathering will use up the excess CO2 thereby cooling the climate. If global temperature cools as a result of some astronomical forcing or tectonic/ocean circulation effect, the lower temperatures will result in lower rates of chemical weathering. Decreased weathering means less CO2 being drawn from the atmosphere by weathering reactions, leaving more CO2 in the atmosphere to increase temperatures. If more rocks become available for rapid weathering as a result of mountain uplift the enhanced weathering will draw down atmospheric CO2 and decrease global temperatures. But the decreased temperatures will slow reaction rates, thereby using less CO2, thus allowing temperatures to moderate.