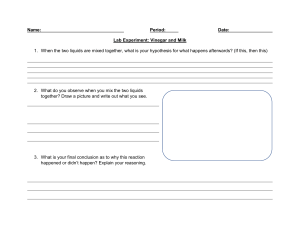
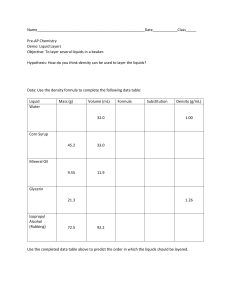
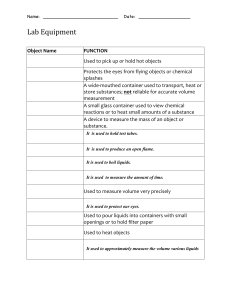
What was your liquid and what was its pH? Lemon Juice with a PH of about 2 Was your liquid acidic, neutral, or basic? What does that mean in terms of OH- and H+ concentration? My Liquid was acidic due to it being below 7. Due to its high acidity it has a high concentration of hydrogen ions and less of OH-. Name two liquids that were acidic and their approximate pH's. Name two that were basic and their approximate pH's. Two Liquids that were acidic are Gastric Acid with a pH of about 1 and Orange Juice with a pH of about 3. Two liquids that are basic are Baking soda with a pH of about 9 and Ammonia Solution with a pH of about 11. What were the most neutral 2 or 3 liquids? Pure water , Human Blood Do you notice any trends or can you make any generalizations about where certain types of liquids fall on the pH scale? Its as if the heavier morse dense liquids are higher on the pH scale where the lighter less dense liquids are lower on the pH scale. Also most consumable liquids tend to be acidic. You can also see the slight trend when it comes to [H+] and [OH-] when it comes to evaluating the pH.