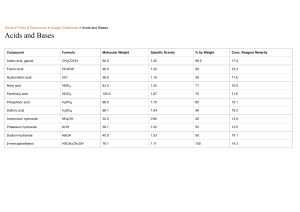
Name: ___ANSWER KEY____________________________ Class: ______ Date: _____________________ Exam #10 Review: Acids, Bases, and pH 1. Know the properties of acids and bases. What are their similarities and differences? Acids Bases Taste sour Taste bitter React with metals to form hydrogen gas Feel slippery +1 Contain a hydrogen ion (H ) Contain a hydroxide ion (OH-1) pH value less than 7 pH value greater than 7 BOTH change colors of indicators react with each other to form salt and water conduct electricity when dissolved in solution (electrolytes) 2. Know the rules for naming acids (binary acid and oxyacid) and name the following below: Binary acids (only 2 elements): hydro – element – ic acid REMEMBER: Oxyacids (contain a polyatomic ion): NO “HYDRO” IN THE NAME “I ate something icky, -ate ion -ic acid all nite I was nauseous” -ite ion -ous acid a. HCl hydrochloric acid d. HNO3 nitric acid b. HF hydrofluoric acid e. H3PO4 phosphoric acid c. HClO2 chlorous acid f. H2SO3 sulfurous acid 3. Know the rules for naming bases and name the following below: Element + hydroxide a. NaOH sodium hydroxide c. Al(OH)3 aluminum hydroxide b. LiOH lithium hydroxide d. Ba(OH)2 barium hydroxide 4. How are Arrehenius acids and bases defined? Acids – produce H+1 ions in aqueous solutions Bases – produce OH-1 ions in aqueous solutions 5. How are Bronsted-Lowry acids and bases defined? Acids – donate (give away) a H+1 ion (proton) Bases – accept (take in) a H+1 ion (proton) 6. Show how the following acids and bases ionize: a. HCl → H+1 + Cl-1 b. NaOH → Na+1 + OH-1 c. H2CO3 → 2H+1 + CO3-2 d. Ba(OH)2 → Ba+2 + 2OH-1 7. If it is a strong acid or base, how much do they ionize in solution? STRONG acids & bases COMPLETELY ionize (all break apart) in a solution 8. If it is a weak acid or base, how much do they ionize in solution? WEAK acids & bases do NOT completely ionize 9. What is a neutralization reaction? What products are always produced? Acid reacts with a base always produces water and a salt 10. Complete the following neutralization reaction and what type of salt is made: SA SB H2SO4 + KOH → _____H2O + K2SO4________________________________ Ionization: 2H+1 + SO4-2 + K+1 + OH-1 SA HBr + SB Sr(OH)2 → Type of Salt: _____neutral______ ______H2O + SrBr2____________________________________ Ionization: H+1 + Br-1 + Sr+1 + 2OH-1 Type of Salt: _____neutral______ 11. How can you tell if the salt produced in a neutralization reaction is going to be acidic, neutral, or basic? Compare strength of acid and base that reacted (if not on list, then it is WEAK) STRONG acid + STRONG base = NEUTRAL salt WEAK acid + WEAK base = NEUTRAL salt STRONG acid + WEAK base = ACIDIC salt the stronger one WEAK acid + STRONG base = BASIC salt wins! 12. What is pH? What ion concentration is measured by the pH scale? How acidic or basic a substance is Measures concentration of hydrogen ions (H+1) 13. What are indicators? Solutions that change colors based on the pH (different colors identify if acid or base) 14. Determine if the following are acids or bases: a. pH = 7 neutral c. pH = 3 acid b. pH = 2 acid d. pH = 8 base