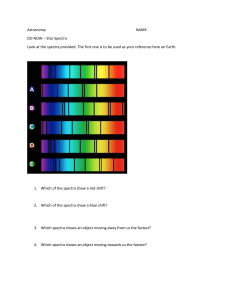
A-Level Chemical Analysis Use a combination of IR Spectroscopy, Mass Spectrometry, NMR Spectrometry and 1H-NMR Spectrometry to identify the following 11 unknown substances 13C- For each substance the spectra are given in the following order: 1. IR 2. MS 3. 13C-NMR 4. 1H-NMR You may find that you need to look multiple times at the spectra and go back and forth between them. Sometimes peaks overlap and some are easier to read than others. However the following order of steps is an effective approach to start from: 1. Use the IR spectra to identify any possible functional groups 2. Use the MS data to determine the relative molecular mass of the unknown substance 3. Subtract the mass of the functional group/s from the molar mass 4. Use this value to determine the number of carbon atoms and then the number of hydrogen atoms 5. Use the 13C-NMR to determine the number of carbon environments 6. Use the 1H-NMR to determine the number of hydrogen environments 7. Using the information from the previous 6 steps propose a name and structure for the unknown chemical SUBSTANCE 1 (EXAMPLE) The group here at 3300 indicates an alcohol If we take 17 (the alcohol, -OH) from 60 we get 43 So we could have 3 carbons (36) and 7 hydrogens (43) as well at the –OH Why 3? Well 4 would be 48 (too much) and 2 would be 24 (too little / too many hydrogens) The molar mass is 60 We have 3 lines so 3 different carbon environments for our 3 carbon atoms Use the data booklet to give you an idea of potential carbon environments 1H-NMR is often the hardest to make out the detail It looks like we have • a quartet • 2 triplets The only molecule that fits all these criteria from the 4 spectra is propan-1-ol SUBSTANCE 2 The molar peak is hard to see here – these are all proper laboratory spectra and you will need to be flexible with your thinking Spectra you receive in the exam will always be much clearer and less ambiguous As long as you use the MS to work out the number of carbons it won’t matter if you, for example, used 59 or 60 SUBSTANCE 3 Shift(ppm) A B C D E 3.63 2.24 1.53 1.39 0.94 Don’t worry if you think there are more environments than you can see Two in this example have overlapped SUBSTANCE 4 SUBSTANCE 5 SUBSTANCE 6 SUBSTANCE 7 SUBSTANCE 8 SUBSTANCE 9 SUBSTANCE 10 SUBSTANCE 11 Answers 1. Propan-1-ol (example) 2. Propan-2-ol 3. Butan-1-ol 4. Ethanoic acid 5. Propanoic acid 6. Propanone 7. 2-methyl-2-butene 8. 1-chlorobutane 9. Pentane 10. Methyl ethanoate 11. Ethanal All spectra from: SDBSWeb : http://sdbs.db.aist.go.jp (National Institute of Advanced Industrial Science and Technology, 14.03.2017)