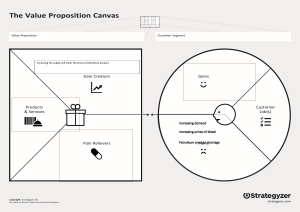
INTRODUCTION Noriel: The demand for biodiesel increases and has been considered one of the alarming issues due to the industrialization, urbanization, and population growth of the world. Based on an article published by Foreign Agricultural Service (FAS), the demand was estimated to increase up to 250 million liters. Additionally, the Biofuels Act of 2006 in the Philippines was implemented requiring that biofuels should be combined with commercially available fuels. Meanwhile, domestic producers can only meet half of the bioethanol demand. Therefore, there is a need for alternative sources of biodiesel. Shena: The possible raw materials for biodiesel production are oil extracted from vegetables, animal fats, algae, and fungi. Align with this, there is a high probability of competition between food and fuel which may result in a price increase for both biodiesel and food-derived oil. Thus, there is an increasing interest in finding alternative feedstock for biodiesel fabrication. Almira: Align with this, waste cooking oil (WCO) is also a source of triglyceride, showing a potential competition to virgin oils because of the price difference. The utilization of waste cooking oil promotes the reduction of its disposal concern. The demand for biodiesel is high as well as the amount of waste cooking oil produced, it can reduce the scarcity of biodiesel. As a result, the WCO-derived biodiesel answers three problems: pollution control, food, and energy security. Bern: The most renowned process of synthesizing biodiesel from WCO is known as transesterification. It includes the reaction of oil and alcohol in the presence of a catalytic medium. The important parameters to be considered for the said process are alcohol and oil type, catalyst type and amount, and the ratio of oil to alcohol. With this, the reaction can be carried out using a homogeneous or heterogeneous catalyst and the selection of the most effective approach varies on the oil's FFA level. Noriel: Unfortunately, homogeneous catalysts might result in soap production, gel formation, produce water when dissolved in an alcohol reaction and absorb moisture while being stored. As a result, interest is increasing in different heterogeneous catalysts because it is non-corrosive, can be reused, can be easily separated from the product, and can be applied in continuous processes. Hydroxyapatite (HAp) is a heterogeneous catalyst derived from calcium phosphate which has the capability to speed up the transesterification process for biodiesel production. Shena: Fishbone is considered food waste and has caused several environmental concerns such as organic odor and disposal issues. On the other hand, it is a natural calcium source that can be utilized in deriving calcium phosphate, which is prominent in the making of hydroxyapatite Almira: In specific, yellowfin tuna (Thunnus albacares) is abundant in tropical countries and widely consumed in the Philippines. Its fishbone contains high calcium and phosphor which makes it notable for hydroxyapatite synthesis. In this study, fishbones of yellowfin tuna (Thunnus albacares) will be used as a source of hydroxyapatite which will act as a heterogeneous catalyst for the transesterification of waste cooking oil to biodiesel. STATEMENT OF THE PROBLEM Bern: Environmental concerns related to food wastes result in the degradation of ecological quality. For this reason, there is a need for alternative applications where it can be recycled for better use. The demand for biodiesel is continuously increasing. The common raw materials for biodiesel production are vegetables and animal oil, however, it results in a dilemma between food and fuel. Noriel: An alternative to this, waste cooking oil has qualities suitable to be a source of biodiesel. However, transesterification involving WCO is a slow process, hence there is a need for a catalytic medium. Shiena: The common catalyst used is homogeneous, which is non-renewable, and is not feasible to be recovered. Thus, researching a more sustainable catalyst is necessary. This study generally aims to synthesize hydroxyapatite which is a heterogeneous catalyst from waste fishbone of yellowfin tuna. In specific, it aims to answer the following questions: *read slide* NULL HYPOTHESES Almira: In this study, the following null hypotheses will be tested: *read slide* SIGNIFICANCE OF THE STUDY Bern: In this study, the researchers will synthesize hydroxyapatite from fishbone, which will be a great potential catalyst for WCO-based biodiesel production in order to meet and to sustain the increasing demand for this type of fuel. In particular, significance of this study lies in the following beneficiaries: Noriel: To the Energy Regulatory Agencies. The study may benefit them through widening of the energy sources array, specifically their origin, and process of production. Noriel: To the Community. They may have an insight on the production process of biodiesel, its benefits and effects, and energy consumption. Shiena: To the Researchers. It would be beneficial in terms of information dissemination of renewable sources of energy which is also valuable in terms of human life and environment. Aside from the awareness, the researchers may contribute the outcome of the project to the local agencies whose focus is on sustainable energy, environment, and health. Amira: To the Future Researchers. This study may include information that benefits future researchers, such as usage of the local wastes, catalysts, and biodiesel production. Also, this study may be used for future references as methodology improvement and outcome quality enhancement. SCOPE AND LIMITATIONS Bern: The study only focuses on the synthesis of a natural and renewable heterogeneous catalyst, hydroxyapatite, from waste fishbones and its utilization for WCO-based biodiesel production. Moreover, the calcium-source samples will only be limited to waste fishbones, specifically from yellowfin tuna which will only be gathered from the local markets and households in the province of Batangas. Meanwhile, the waste cooking oil samples will only be gathered from the restaurants, fast food chains, and households also of the specified province. The catalyst and biodiesel produced will only be tested according to the given and specified parameters. Noriel: The data in this study will be limited by the availability of the formerly specified fish species and by the quantity of the samples that will be gathered from the local province. There is a rare possibility that these species are seasonal and limited for some causes such as in crisis, short in supply, and affected by local disasters and calamities. Moreover, the quantity of waste cooking oil would limit the study in a way that some restaurants and households reused their cooking oil. CONCEPTUAL FRAMEWORK Shiena: The conceptual framework of the study is hinged on the concepts that convert waste cooking oil to biodiesel through association of a heterogeneous catalyst, specifically hydroxyapatite.For this study, the input variables for WCO-based biodiesel production are waste cooking oil, waste fishbones, potassium hydroxide solution, and phosphoric acid solution. These were vital for the processes involved which are preparation and purification of the raw materials, catalyst synthesis, transesterification process, design of experiment, and further data analyses.The expected output of this study were fishbone-derived hydroxyapatite, WCO-based biodiesel, glycerol, a model equation, statistical results, and the optimized condition of transesterification among the predetermined runs. CONCEPTUAL LITERATURE Almira Biodiesel Biodiesel is a clean-burning renewable fuel substitute for traditional petroleum-based fuels. There are 3 different feedstocks used in biodiesel production: first-generation biofuel-feedstocks are extracted from crops which are used for food consumption. Second-generation biofuel-feedstocks were synthesized from crops but from those that were not used for human consumption. Third-generation biofuel-feedstocks are from waste or used oils. Accordingly, the yielded biodiesel should conform with the requirements set by the United States Environmental Protection Agency (EPA), in accordance with section 211 of the Clean Air Act, for fuels and fuel additives and the standard of the American Society of Testing and Materials Bern WCO New developments in the technology that transforms used cooking oil into high-quality refined oil have encouraged the expansion of the market, as a whole. Almost 15 million tons of cooking oil are generated annually, an enormous amount that, if converted, could partially meet the need for biodiesel around the world. The generation of biodiesel from WCOs enables savings of 96% of fossil energy and 21% of crude oil. WCOs can be turned into biodiesel at very high yields per kilogram. Transesterification process alters the physicochemical characteristics of WCO to make it better suited for compression ignition engines. Prior to biodiesel production, the properties of the WCOs should be determined to assess if these can have an adverse effect on the production of biodiesel. Shiena YELLOWFIN TUNA The Yellowfin Tuna (Thunnus albacares) is characterized by its torpedo-like shape, dark metallic blue dorsal area, yellow sides, and silver belly. The two species with the highest catch rates in the Philippines are yellowfin and skipjack tuna. In the study of Nam et al. in 2019, fishbone of 4 species including tuna was compared to assess the amount of HAp it can synthesized. Table 2.6 shows that the samples are mostly composed of calcium and phosphorus, with an average Ca/P molar ratio of roughly 1.80. This ratio exceeds the stoichiometric one found in human bone, which is 1.67. Almira HYDROXYAPATITE In the fields of energy and environmental protection, as well as the production of chemicals and refinery operations, there is a growing interest in the creation of a new generation of solid catalysts. Moreover, an inexpensive mineral catalyst for the production of biodiesel is hydroxyapatite. When synthetic and natural hydroxyapatite are compared, Natural hydroxyapatite differs from synthetic hydroxyapatite in a way that it possesses disordered nanostructures, nonstoichiometric composition, and a lower hydroxyl content. Thus, it is reasonable to anticipate that biological hydroxyapatite will yield better results as a functional material if its nanoscale properties are maintained. For the case of biodiesel production, an appropriate quantity of catalyst can enhance the reaction's efficiency and minimize saponification Bern TRANSESTERIFICATION In this process, triglycerides from oils or fats are reacted with alcohol, usually methanol, to form fatty acid methyl esters (FAMEs), also known as biodiesel, and glycerol. The reaction requires three moles of alcohol for every one mole of triglyceride to produce one mole of glycerol and three moles of FAMEs. Methanol is favored due to its low cost, chemical properties (shortest alcohol chain), and physical properties (polarity) in chemical reactions. Based on Figure 2.10, in transesterification, ester bonds in triglycerides are broken and replaced with alcohol to yield fatty acid methyl esters (FAMEs). It is important to note that a higher ratio of methanol to oil usually results in a faster reaction rate and higher production of FAMEs but it may also cause an increase in the formation of glycerol and soap byproducts. Noriel REFLUX Reflux method is useful in chemical synthesis because it allows the reactions to be carried out at higher temperatures and for longer periods of time rather than simple heating. In this case, Fishbone waste was used as a source of Calcium, and the reflux method was used to produce hydroxyapatite by bonding the Calcium with Phosphate from the solution. Shiena 3-Factor It is used to create a mathematical connection between process parameters and their corresponding response. Its main objective is to identify the optimal value combination of these parameters through statistical analysis. According to Daniyan et al., in terms of biodiesel production, the use of Central Composite Design (CCD) has the potential to benefit the production such that it can aid in biodiesel yield prediction and achieve a high conversion rate of vegetable oil to biodiesel, as well as providing a means for process control. RESEARCH LITERATURE *basahin na lang asa table* METHODOLOGY Almira Before the actual conduct of the experiment, a pre-laboratory will be conducted to ensure the accuracy of set levels for each factor. From the result of the pre-laboratory, several considerations will be made to obtain a cost-effective product but will also ensure its quality. Bern Collection of Raw Materials For the collection of waste fishbone and WCO, the researchers will be dealing with partnerships within the local restaurants, fast food chains, and local market. The collected materials will be stored properly in an enclosed container to avoid additional contaminations. Noriel Purification of WCO Food particles and debris will be filtered out from the waste cooking oil (WCO) through the filtration process. To remove the water component of the oil, it will be heated in an oven at a temperature of 110 ℃ for about half an hour. The remaining water after heating was allowed to settle at the bottom, after that the water will be removed through decantation. After this, purified WCO will be produced. After purification, samples will be sent to the DOST testing center to assess its viscosity, moisture content, acid value, saponification value, and free fatty acid (FFA) content. Shiena Preparation of Waste Fishbone To free the samples from debris and fats, they will be boiled in water for ten (10) minutes at a temperature of 100 ℃. The boiled fishbones will be dried in an oven at a temperature of 90 ℃ for about an hour and 40 minutes and will proceed through the process of crushing and pulverization using mortar and pestle. Further drying of the powder will be done using a dry-heat oven for 24 hours at 60 ℃. To obtain the fine powder, high-energy ball milling will be utilized for another 24 hours. Milled fishbone powder will be dried once more using a microwave oven at a temperature of 60 ℃. Once dried, it will be placed on a desiccator to maintain its dry condition. Almira The catalyst will be synthesized through reflux method Deproteinization Starting with deproteinization, A 5% KOH solution will be utilized to deproteinize the powder samples. The solution will be used to create a strong base solution that is efficient in the hydrolyzation of the calcium and protein in the fishbones. The powder sample and the KOH solution will be placed in a 500 mL beaker and will be boiled at a temperature of 130 ℃ for 18 hours. Bern Reflux After deproteinizing the samples, it will proceed in refluxing. The reflux will be utilizing H3PO4 (weak acid) with 2.33M as a source of PO43-, that would be the donor ion for hydroxyapatite synthesis. The solution will be placed in a 500 mL beaker and will be boiled at a temperature of 130 ℃ for 12 hours. Noriel Calcination The refluxed sample will now proceed to the calcination process. It will be done using a high-temperature furnace at 900 ℃ for 8 hours. After this, the resulting product will be the hydroxyapatite. After calcination, samples of yielded hydroxyapatite will be sent to DOST testing center for characterization using X-ray Diffraction (XRD), scanning electron microscopy (SEM), Transmission Electron Microscope (TEM) and Fourier Transform Infrared Spectrometry (FT-IR) Shiena Transesterification It will be done in a three‐necked 500 ml round‐bottom flask immersed in a water bath, to control the temperature in the hot plate. The middle neck is used to insert a water-cooled condenser and the other neck is fixed with a thermometer to control the reaction temperature. Waste fishbone-derived hydroxyapatite was utilized in this procedure as a catalyst loading. Then, oil to methanol ratio and HAp concentrations were measured simultaneously taking into consideration the process parameters. Then, it was combined in a shake flask. The shaker's settings remained constant at a reaction temperature of 65 °C, a reaction time of 4 hours, and a speed of 300 rpm. The samples in the shaking flask were centrifuged at 4000 rpm for 10 minutes after transesterification was finished. Following that, the samples were filtered through filter paper to get rid of any contaminants and catalyst loading. Shiena Reusability of Catalyst It will be examined based on the amount of biodiesel yield at the optimal operating setup. The filtered catalyst will be reused until there is a significant change in the duration of the transesterification process and the amount of the yielded biodiesel. Almira Biodiesel and glycerin separation The biodiesel (FAME) and glycerol were separated after filtering by pouring the mixture into a separator funnel. Moreover, the samples were divided into two layers after a short while. After that, glycerol made up the bottom layer while biodiesel made up the top layer. The glycerol layer was then taken off and thrown away. Then the remaining layer will be the unrefined biodiesel Bern Washing of Methyl ester The obtained biodiesel will be refined by washing because it will still contain trace amounts of methanol and glycerine. Additionally, the separatory funnel will be filled with warm water, which will then be added and will slightly be agitated to allow it to settle. The methyl esters will be effectively separated from the water, which will then be drained out of the bottom. Moreover, the process will be done several times until the water has a pH of 6-7 and no soap bubbles are visible on it. Sample of the yielded biodiesel will be sent to DOST testing center to assess specific parameters in this study and the standard of ASTM and DOE Noriel Yield Analysis The biodiesel yield will be computed using the formula: Biodiesel yield (%) = (volume of biodiesel / volume of WCO) * 100 After all of this, we will have the catalyst reusability analysis, biodiesel yield properties, and yield analysis Bern Design of Experiment Response Surface Methodology (RSM) will be used to model and to optimize the transesterification process parameters. The detailed analysis will be generated utilizing Design-Expert version 12. Conversion of biodiesel and glycerol will be the process response while the reaction variables are contact time, methanol to oil ratio, HAp dose, and temperature. A total of 78 runs will be utilized in this study. Almira Statistical Treatment of Data In determining the statistical evaluation of the percent yield of biodiesel in varying conditions multiple analysis of variance (ANOVA) will be used while one-way ANOVA will be utilized to evaluate the reusability of HAp. In addition, T-test will be used in the comparison of the yielded biodiesel and the B100.