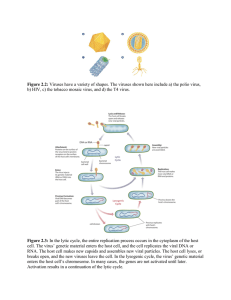
UNIVERSITY OF SANTO TOMAS FACULTY OF PHARMACY | DEPARTMENT OF MEDICAL TECHNOLOGY CLINICAL VIROLOGY TERMINOLOGIES AND BASIC PRINCIPLES ֍ VIRUS § § § Obligate intracellular organism that contain only kind of nucleic acid as their genome The nucleic acid is encased in a protein shell (CAPSID) which may be surrounded by a lipid membrane (ENVELOPE) Smallest infectious agent (from 20 to 300 nm in diameter) Smallest Virus : Picornaviridae Largest Virus : Poxviridae Highly Dependent on hosts Viruses do not have a metabolic system ֍ VIRION § Complete viral particle § A virus with its genome enclosed by the capsid (NUCLEOCAPSID) and its envelope § In certain instances, synonymous to NUCLEOCAPSID Applicable to NAKED Viruses such as the Papillomavirus and the Picornavirus ֍ GENOME § Genetic material composed of DNA or RNA § May be Single Stranded (in most RNA virus) or Double Stranded (in most DNA virus) ֍ POLARITY § The genome possessed by the virus may be of the messenger type (Sense or + strand) or a complimentary strand (antisense or – strand) § Sense / + Strand Nucleic Acid runs from 5’ to 3’ hence +ssRNA can readily be translated into viral proteins § Antisense / - sense Nucleic Acid runs from 3’ to 5’ (complimentary to a mRNA) which cannot be translated immediately by the host cell Needs RNA-dependent RNA polymerase packaged inside the virion The RNA polymerase will transcibe the antisense into a sense strand before it can be translated by the host cell ֍ PEPLOMERS § Viral-encoded glycoproteins found as projections on the surface of envelopes ֍ HELICAL VIRUS § Shaped like a hollow protein cylinder which may be rigid or flexible ֍ ICOSAHEDRAL VIRUS § Polyhedron with 20 triangular sides (capsomeres) ֍ COMPLEX VIRUS § Capsid symmetry but not purely icosahedral nor helical § Example are Bacteriophages and Poxviruses ֍ ECLIPSE PERIOD § Infectious virion is not seen VIRAL REPLICATION ֍ For replication to happen, the viral genome must be able to produce a functional mRNA to be able to synthesize viral proteins by the host cell protein-synthesizing machinery. ֍ Outcomes § Productive infection – successful production of PROGENY ü Lytic Infection – progeny released via cell lysis ü Persistent infection – either no lysis involved or there is a reservoir cell § Nonproductive Infection ü Latent infection – maintains viral genomes stably in host cell; no progeny produced ü Transforming infection – no progeny but the viral genome becomes chromosomally integrated; infected cells become cancerous cells ü Abortive infection – defective virus / no replication occurred ֍ ADSORPTION § First step in the viral replcition cycle § Virus recognizes a suitable host cell § Specific binding with capsid proteins and carbohydrate receptor of host cell § List of Host Receptors used by Specific Viruses ü Parvovirus B19 : P-antigen (globoside) ü Adenovirus : Coxsackie-Adenovirus Receptor (CAR) ü EBV : C3D (cr2 or CD21) ü HIV : CD4 receptor ü HSV1 : Fibroblast Growth Factor ü Influenza 1 : Sialic Acid ü Rubeola : CD46; Signaling Lymphocytic Activation Molecule (SLAM) ü Rhabdovirus : Acetylcholine Receptor; CD56 ü Polio : CD155 [Poliovirus Receptor (PVR)] ü Rhinovirus : ICAM-1 ü Vaccinia : Epidermal Growth Factor ֍ PENETRATION VIROLOGY | 1 UNIVERSITY OF SANTO TOMAS FACULTY OF PHARMACY | DEPARTMENT OF MEDICAL TECHNOLOGY Entry of Virus into the Host Cell Mechanisms of Entry ü Direct Fusion • One of the mechanisms used by enveloped viruses • The viral envelope and the cell membrane of the host fuse causing the viral nucleocapsid to be directly delivered in the cytoplasm leaving the viral envelope on the plasma membrane • Ex. RETROVIRUS ü Receptor-Mediated Endocytosis • Used by either naked or enveloped viruses • Following the engagement of viral particles on the receptor, the virus particlereceptor complex triggers the endocytosis by forming a coated pit on the plasma membrane, leading to endosome formation. As a result, the virus particle becomes located inside the endosome. • Enveloped viruses will have its envelope fused with the endosomal membrane. • Naked viruses will trigger endosomal membrane lysis via its capsid proteins ü Translocation ü Injection of Viral Material (use several tail fibers and lysozyme) ֍ UNCOATING § Disassembly of the virion § Exposure of the viral genome ֍ TRANSCRIPTION and TRANSLATION § Transcription is the biosynthesis of mRNA from a DNA template using DNA-dependent RNA polymerase (synthesizes RNA in 5’-3’ direction from the template DNA strand which runs in 3’-5’ direction) § RNA viruses no longer need a DNA Template especially the +/sense ssRNA which serve as messenger RNA and are ready for translation ü -/antisense RNA needs RNA-dependent RNA polymerase to convert it into sense strand (enzyme packed in the virion) § Translation is the biosynthesis of amino acids from codons leading to synthesis of peptides/proteins ü Formation of viral proteins § Transcription and Translation occurs first before replication. ü Production of viral proteins that are necessary for replication to occur ֍ DNA REPLICATION § Most dsDNA are able to use host enzymes to express viral genes and replicate viral DNA § Replicate via dsDNA (ssDNA need to synthesize a complementary strand to convert the genome into dsDNA) § Require DNA-dependent DNA polymerase § Each viral DNA has a specific origin of replication § Proteins that initiate DNA replication bind to the origin ü Helicase – unwind the double helix ü ssDNA binding protein – keep the strands apart ü DNA polymerase – for transcription § § clinical.virology | 2 UNIVERSITY OF SANTO TOMAS FACULTY OF PHARMACY | DEPARTMENT OF MEDICAL TECHNOLOGY One of the daughter strands is the leading strand and the other is the lagging strand, synthesized as Okazaki fragments, which become joined by a DNA ligase. § After a dsDNA molecule has been copied each of the daughter molecules contains a strand of the original molecule. This mode of replication is known as semi-conservative, in contrast to the conservative replication of some dsRNA viruses. dsRNA replication § similar to dsDNA in which the helix is unwind ssRNA REPLICATION § Translation occurs first to produce the enzyme replicase which is needed for replication ü - sense are transcribed to + sense § ssRNA are replicated by synthesis of complementary strands of RNA that are then used as templates for synthesis of new copies of the genome ASSEMBLY § Progeny and Viral proteins form virion EXIT § Virion exit the host cell then infect a new host cell § ֍ ֍ ֍ ֍ SPECIMEN COLLECTION ֍ Specimens should be collected as soon as possible after the onset of symptoms § Likelihood of obtaining + results : first 3 days after onset of symptoms ֍ Viral Transport Media (VTM) § Required for Respiratory samples, swabs, and tissue samples because they dry out easily. § Formulations typically consist of a buffered salt solution such as Hanks’ balanced salt solution buffered with HEPES to maintain a neutral pH, protein-stabilizing agents such as bovine serum albumin or gelatin, and antimicrobials to prevent bacterial and/or fungal overgrowth. § The inclusion of sucrose in the media serves as a cryoprotectant to maintain the integrity of viruses if specimens are frozen (−70°C or lower) for prolonged periods. § A sample collection and transport device that contains an absorbent matrix for drying of specimens, ViveST (ViveBio, Norcross, GA), has been used primarily for preservation of NA at ambient temperature, although some viruses remain viable for up to 7 days with the addition of fetal bovine serum ֍ Swabs that are not used include calcium alginate, charcoal, and those with wooden shafts ֍ Dried samples for Nucleic Acid Testing are acceptable ֍ Transport Conditions § Typically, specimens for viral culture should be transported to the laboratory promptly, ideally within 2 to 4 hrs of collection § The preferred transport temperature is 2 to 8°C, except for blood specimens, which can be transported at ambient temperature. § Viability is not a requirement for Ag or NA detection methods; therefore, transport time may be less significant, unless degradation of intact cells or viral NA is a consideration. ֍ Selected Specimens § Amniotic Fluid § NA detection is the most commonly used method § NAs from samples should be extracted as soon as possible and stored at 2 to 8°C for up to 48 h or frozen at −70°C or lower if testing is delayed beyond 48 h § Congenital CMV : 21-23 wks of gestation for most accurate prenatal dx § Maternal VZV during 1st/2nd Trimester – VZV DNA is detectable § Blood § EDTA and ACD are the preferred anticoagulants for obtaining plasma for NA testing, as heparin is inhibitory to many NA amplification chemistries § Whole blood used for NA amplification must be processed to remove inhibitors of DNA and RNA polymerases such as heme and metabolic precursors of heme. § When serum is required for serologic or molecular testing, 4 to 8 ml of blood is collected in a serum separator tube § For virus isolation, 5 to 10 ml of anticoagulated blood (EDTA or heparin) should be collected by venipuncture and transported to the laboratory at room temperature. • Processing of the specimen for leukocyte fractionation should occur within 8 h. • Whole blood should not be used to inoculate cell cultures because of toxicity caused by red blood cells. The buffy coat fraction may also contain erythrocytes. • For recovery of CMV in culture, the shell vial technique is more rapid and sensitive than conventional tube culture § Bone Marrow § Bone marrow specimens should be stored at 2 to 8°C prior to NA extraction. Freezing and thawing of bone marrow lyses red cells, causing release of heme, a known inhibitor of PCR § CSF § Transport and store for up to 48 h at 2 to 8°C, and freeze at −70°C or lower for longerterm storage. § NAs should typically be extracted prior to testing since CSF contains globulins, cell-derived proteins, and other uncharacterized substances that inhibit the activity of thermostable polymerases used in PCR § Eye § swabs or scrapings of the conjunctiva, corneal scrapings, and vitreous and aqueous fluids. § Corneal scrapings and swabs of the cornea are best collected and placed in VTM § Direct testing of these surgically obtained fluids has resulted in PCR inhibition; therefore, the original specimen must be extracted. § Genital clinical.virology | 3 UNIVERSITY OF SANTO TOMAS FACULTY OF PHARMACY | DEPARTMENT OF MEDICAL TECHNOLOGY § § § § § § § The most common viral causes of genital lesions are HSV-1 and -2, which are easily detected by direct Ag (DFA) methods, NATs, or culture Oral Respiratory § The primary site of replication for many respiratory viruses is the ciliated epithelial cells of the posterior nasopharynx and, to a lesser extent, the anterior nares and oropharynx. § Nasal washes can yield high rates of respiratory virus detection by NAT with minimal patient discomfort compared with swab, aspirate, and brush sampling § Swabs for respiratory virus testing should be polyester, Dacron, or rayon with plastic or aluminum shafts. A swab of the posterior nasopharynx typically yields more virus than a swab of the anterior nares or throat. • Woodenshaft swabs may contain substances that are toxic to cultured cells. • Calcium alginate swabs should not be used since they may impair recovery of enveloped viruses, may interfere with fluorescent-antibody tests, and are inhibitory to some NATs. • Flocked swabs, made from nylon fiber using a proprietary spray-on technology, are designed for optimum specimen absorption and release and have been shown to collect more respiratory epithelial cells than conventional rayon swabs for DFA testing of respiratory viruses. • Polyurethane foam-tipped swabs provide an alternative to nylon or Dacron swabs for sampling of the anterior nares in patients who might be at risk for bleeding. Skin § Vesicular lesions from HSV Stool and Rectal Swabs § Stool - optimal specimen for identification of viruses causing gastroenteritis such as rotavirus. § Fresh stool specimens can be stored at 4°C for 2 to 3 days if they are not tested immediately after collection. For prolonged storage, specimens should be kept frozen, preferably at −70°C or lower. Tissue / Biopsy § Formalin-fixed tissue is unsuitable for viral isolation and may affect the performance of NATs § For viral isolation from tissue, it is recommended to prepare a 10 to 20% (wt/vol) homogenate using VTM as a diluent from small or minced tissue fragments that have been aseptically ground in a tissue grinder Urine § Collect urine specimens as soon as possible after the onset of illness or when congenital or perinatal infection is first suspected. § For NATs, ambient storage of fresh, unprocessed urine should be minimized since the low pH and high urea content rapidly denature DNA and RNA § Prior to inoculating cell culture, urine can be filtered (0.45-μm pore size) or centrifuged (1,000 × g for 10 min) to remove bacteria and debris; the pH can be neutralized with sodium bicarbonate (7.5% solution) to reduce toxicity TECHNIQUES ֍ Light Microscopy – visualize cytopathic effects § CMV : Owl’s Eye Inclusion Body § HPV : Koilocytosis, perinuclear halo ; wrinkled-prune nucleus § Molluscum contagiosum : Henderson-Paterson Bodies § Rhabdovirus : Negri Bodies § HSV 1 : Cowdry Type A § Adenovirus : Cowdry Type B; grape-like clusters § Poxviruses : Guarnieri Bodies § Rubeola : Dawson Bodies / Warthin Finkeldey/ Multinucleated giant cells with syncytia § Yellow Fever : Councilman Body ֍ Cell Culture – gold standard § Maintained and incubated for 1-4 weeks § Primary Cell Line (Human Embryonic Kidney / Rabbit Kidney / Monkey Kidney) § Sensitive to influenza, parainfluenza, mumps, enterovirus, and adenovirus § Diploid Cell Lines (Human Diploud fibroblasts/ W1-38 / mrc-5) § 20-25 passages § Continous/ Heteroploid / immortal cell lines § HeLa – cervical carcinoma § Hep-2 – laryngeal ca § KB – nasopharyngeal § A-549 – lung ca ֍ Shell Vial – modified conventional cell culture TAXONOMY AND CLASSIFICATION ֍ BALTIMORE CLASSIFICATION Group I II III Classification dsDNA ssDNA dsRNA IV (+) ssRNA Examples Herpesviridae, Poxviridae, Adenoviridae, Papovaviridae Parvoviridae Reoviridae Caliciviridae, Flaviviridae, Coronaviridae, Astroviridae, Picornaviridae, Togaviridae clinical.virology | 4 UNIVERSITY OF SANTO TOMAS FACULTY OF PHARMACY | DEPARTMENT OF MEDICAL TECHNOLOGY V (–) ssRNA VI VII RNA RT DNA RT Bunyaviridae, Arenaviridae, Paramyxoviridae, Orthomyxoviridae, Rhabdoviridae, Flioviridae Retroviridae Hepadnaviridae ֍ DNA VIRUSES – replicate in the nucleus § NAKED / NON-ENVELOPED § PARVOVIRUS – single-stranded DNAV; linear NA • Smallest DNA virus § ADENOVIRUS – the only naked dsDNA with a linear NA § PAPILLOMAVIRUS § POLYOMAVIRUS § ENVELOPED § HEPADNAVIRUS § HERPESVIRUS – linear NA § POXVIRUS – largest virus; brick-shaped; replicates in cytoplasm ֍ RNA VIRUSES – replicates in cytoplasm § Sense § CALICIVIRUS § CORONAVIRUS § PICORNAVIRUS § FLAVIVIVIRUS § ASTROVIRUS § TOGAVIRUS § HEPEVIRUS § Antisense § FILOVIRUS § BUNYAVIRUS § ARENAVIRUS § PARAMYXOVIRUS § ORTHOMYXOVIRUS § RHABDOVIRUS § RETROVIRUS DNA VIRUSES GENERALITIES § § § § § § § § All are linear except Papovaviridae and Hepadnaviridae All are double-stranded except Parvoviridae All are naked except Herpesviridae, Hepadnaviridae and Poxviridae All are icosahedral except Poxviridae All replicate in the nucleus except Poxviridae DNA Tumor Viruses: Ex. Human Papillomavirus, EBV, HBV, HHV8, Polyomavirus, SV40 virus, Smallest DNA Virus = Parvoviridae Largest DNA Virus = Poxviridae NAKED DNA VIRUSES ֍ PARVOVIRUSES § Two subfamilies : Parvovirinae and Densovirinae § Parvovirinae infect vertebrates § Densovirinae infect insects § Smallest DNA animal virus § Pathology § Erythema infectiosum / fifth disease • Original 6 Exanthematous Disease ü 1. Measles ü 2. Scarlet Fever ü 3. Rubella ü 4. Dukes ü 5. Erythema Infectiosum ü 6. Erythema Subitum § Childhood exanthem § Polyarthralgia-arthritis syndrome in adult § Aplastic crisis in patients with hemolytic disease § Chronic anemia among the immonocompromised § Fetal death / hydrops fetalis § Icosahedral; nonenveloped § Possess 2 core proteins § VP2 : major protein § VP1 § HUMAN PARVOVIRUS B19 § Erythrovirus genus § Receptor : P antigen (globoside) § Target : erythroid progenitors § Alpha-5-Beta-1 Integrin : coreceptor § NS1 : required for viral replication § MOT • Respiratory route clinical.virology | 5