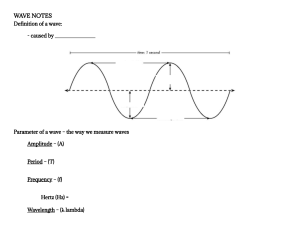
4/5/23, 2:11 PM تسجيل الدخول Cambridge A Level Physics: Definitions إنشاء مدونة إلكترونية Cambridge A Level Physics CIE 9702 free resources by ETphysics Welcome! Study Guide Content Library Equations Definitions Simulations Definitions Topical Questions Recent Posts What Are You Looking For? A combination of markscheme answers and textbook definitions. Search Jump to sections: [AS Chapters][A2 Chapters] Important Resources AS Level Physics Terms & Concepts Instagram Pocket Notes Here's a useful set of flashcards for AS revision: YouTube Online Classes https://quizlet.com/356145378/as-level-physics-definitions-cie-flash-cards/ https://quizlet.com/527383170/cie-as-level-physics-definitions-flash-cards/ Lvl Ch Term Definition Join Discord (ZNotes) Physics Reference Extra Video Library AS 1 Derived Units Some combination of the base units. The base units may be multiplied together or divided by one another, but never added or subtracted. AS 1 Homogeneous Units When each term has the same base units, the equation is said to be homogeneous or ‘balanced’. AS 1 Scalar A quantity that has magnitude/size AS 1 Vector A quantity that has magnitude/size and direction AS 1 Accuracy How close a reading is to its true value. When readings are accurate, the peak / average value moves towards the true value AS 1 Precision Smallest change in value that can be measured by an instrument. OR Spread of values / measurements (scatter between each data is relatively small/ lines are closer together / sharper peak) AS 1 Random errors Readings have positive and negative values around the peak value / values are scattered / wide range. To reduce errors, take several readings to get an average value. AS 1 Systematic Error The average / peak is not the true value / the readings are not centred around the true value. Look/check for zero error to avoid systematic error. AS 1 Uncertainty The range of value within which a measurement is likely to be in. AS 2 Acceleration (vector) Rate of change of velocity. AS 2 Displacement (vector) is the straight line distance between start and finish points (in that direction) / minimum distance AS 2 Distance (scalar) is the actual path travelled AS 2 Free Fall The downward motion of an object under the influence of force of gravity with a constant acceleration (g = 9.81 ms-2). AS 2 Projectile motion Objects acted upon by a force with vector at perpendicular to its horizontal velocity. Asume zero frictional forces. Tracjectory of the object will result in a parabola. AS 2 Speed (scalar) Distance travelled per unit time taken AS 2 Terminal velocity Constant speed of object when resultant force is zero due to large air resistance. AS 2 Velocity (vector) Rate of change of displacement AS 3 Conservation Of Total momentum of (an isolated) system (of interacting bodies) remains constant, provided Momentum there are no resultant external forces (e.g. friction) AS 3 Total momentum and total Kinetic Energy of a system is conserved. Relative speed of Elastic Collisions approach is equal to the relative speed of separation AS 3 Force Rate of change of momentum AS 3 Force It is defined as the rate of change of momentum of a body ► 2011 (1) AS 3 Impulse It is the product of a force & the time during which the force is applied. ► 2010 (1) AS 3 Inelastic Collisions Total momentum of a system is conserved, but the total Kinetic Energy is not conserved. Speed before impact is not equal to speed AS 3 Linear Momentum Product of an object's mass & velocity, with its direction always being the same as the direction of velocity. AS 3 Mass It is a measure of inertia of a body or the property of a body that resists change in motion AS 3 Newton’s 1st Law A body remains at rest or constant velocity unless acted on by a resultant (external) force AS 3 Newton’s 2nd Law The (resultant) force is proportional to the rate of change of momentum AS 3 Newton’s 3rd Law If one body exerts a force on another, it will experience a force by the other body, which is equal in magnitude & opposite in direction. Both forces are the of the same kind. AS 3 Weight Weight is the force due to the gravitational field AS 4 Centre Of Gravity The point on an object at which the entire weight of the body seemingly acts. It is the point at which the Earth actually applies the pull of gravity. AS 4 Density Amount of mass per unit volume of a substance. AS 4 Equilibrium Net / resultant force and moment is zero (OR sum of clockwise moments = sum of anticlockwise moments). If the triangle of forces is ‘closed’ then there is no resultant force https://9702-physics.blogspot.com/p/definitions.html Lab Practical Solutions P3Q1 (1) P3Q2 (1) P5Q1 (4) P5Q2 (18) Past Year Questions Archive ▼ 2022 (4) ▼ June (2) MJ22 P52 Q2 Cepheid Luminosity MJ22 P52 Q1 Magnetic Launcher "Rail Gun" Metal Bar ► March (1) ► January (1) ► 2021 (4) ► 2020 (9) ► 2019 (3) ► 2018 (2) ► 2017 (3) ► 2016 (1) Translate (Equations Will Error) ▼ اختيار اللغة Chatbox 1/8 4/5/23, 2:11 PM Cambridge A Level Physics: Definitions and the object is in equilibrium. AS 4 Moment / Torque Product of the force and the perpendicular distance to the pivot AS 4 Pressure The perpendicular/normal force applied per unit area AS 4 Principle Of Moments The sum of the clockwise moments about a point equals the sum of the anticlockwise moments (about the same point) AS 4 Torque Of A Couple Product of one of the forces and perpendicular distance between forces. (The turning effect caused by two equal & opposite forces when their line of actions are different.) AS 4 Upthrust It is the resultant force on a submerged object due to pressure difference between the higher pressure at the bottom of the object and the lower pressure at the top of the object immersed in a fluid. AS 5 Energy It is the stored ability to do work. AS 5 Work Done Product of a force & the distance moved in the direction of the force. AS 5 Gravitational Energy stored due to height/position of mass Potential Energy AS 5 Internal Energy It is the total of the microscopic Kinetic & Potential energies of particles of a material. AS 5 Kinetic Energy Energy of an object due to its motion. AS 5 Potential Energy Energy stored by an object to do work AS 5 Elastic Potential Energy stored due to deformation or change in shape of an object Energy AS 5 Electric potential Potential energy (stored) when charge moved due to work done in electric field energy AS 5 Power Rate of work done AS 5 Efficiency The fraction of the useful power output obtained from the total power input. AS 6 Brittle Materials Materials which do not undergo plastic deformation. Force proportional to extension until it breaks AS 6 Ductile Materials Materials which undergo plastic deformation after a considerable elastic deformation. Initially force proportional to extension then a large extension for small change in force AS 6 Elastic Deformation Object returns to its original length (zero extension) when load is removed AS 6 The area under such a graph is the work done in stretching a material. For the straight-line Force-Extension portion of the graph, it is a measure of the elastic potential energy stored by the material, Graph provided that the graph for decreasing loads is the same as that for increasing loads. It is also known as strain energy. AS 6 Hooke's Law Force/load is proportional to extension/compression if proportionality limit is not exceeded. AS 6 Necking When a sufficiently large force is applied, localized narrowing occurs at weak points, & the wire eventually breaks at one of these points. AS 6 Plastic Deformation Wire/body object does not return to its original shape / length when load is removed AS 6 Polymeric Materials Materials which can undergo great strain, & deform to a very great degree. E.g. rubber, glass, cement AS 6 Strain Extension over original length (ratio). Stress is the cause & strain is the effect. AS 6 Stress It is the force per unit cross-section area required to stretch a material. AS 6 Ultimate Tensile Strength The maximum force / original cross-sectional area the wire is able to support before it breaks AS 6 Ultimate Tensile Stress The maximum value of stress that an object can sustain before it breaks. AS 6 Young’s Modulus Ratio of stress to strain. AS 7 Progressive wave The transfer or propagation of energy as a result of oscillations / vibrations AS 7 Transverse Waves A wave in which displacement of particles is perpendicular to the direction of wave propagation, resulting in crests & troughs. Transverse waves have vibrations that are perpendicular / normal to the direction of energy travel AS 7 Longitudinal Waves A wave in which displacement of particles is parallel to the direction of wave propagation. Longitudinal waves have vibrations that are parallel to the direction of energy travel AS 7 Wavelength Distance moved by wave energy / wavefront during one cycle of the source or minimum distance between two points with the same phase or between adjacent crests or troughs. AS 7 Frequency (Hz) Number of oscillations per unit time (not per second) AS 7 Period The time taken to complete one oscillation/cycle. Or time between adjacent wavefonts. AS 7 Amplitude Maximum displacement of a particle in the wave AS 7 Displacement Distance (of point on wave) from rest / equilibrium position AS 7 Phase Difference The difference in the relative positions of the crests or troughs of two waves of the same frequency expressed in radians or degrees. AS 7 (Wave) Intensity The intensity of a wave is the energy passing through unit area per unit time. AS 7 (Wave) Speed Speed at which energy is transferred / speed of wavefront. It is NOT the speed with which particles in the wave move. AS 7 Doppler Effect Change in observed frequency when source moves relative to the observer AS 7 electromagnetic waves (a transverse wave) can travel through a vacuum / free space. The Electromagnetic displacement in the case of electromagnetic waves is a variation in the electric & magnetic Waves fields perpendicular to each other. AS 7 Polarisation Oscillations or vibrations are in one direction, perpendicular to direction of propagation. AS 8 Transfer of Energy The transfer of energy is due to a progressive wave, NOT a standing/stationary wave. AS 8 Coherence Two waves with a constant phase difference are said to be coherent. AS 8 Principle of Superposition When two waves of the with similar frequency &amp meet/overlap, the resultant displacement is the sum of the individual displacement of each wave. wondering if I could get the AS physucs notes 2 weeks ago Mushfiq mushfiqurrahman345@gma il.com Mushfiq hello maam 2 weeks ago Mushfiq @ETPhysics 2 weeks ago 1 week ago meer they wont reply bro Cbox name message To donate, send Bitcoin to: Or send tokens of thanks via Paypal! About Me Lifelong learner who loves creating music. Based in Malaysia. Error Report Name Email * https://9702-physics.blogspot.com/p/definitions.html Message * Send 2/8 4/5/23, 2:11 PM Cambridge A Level Physics: Definitions AS 8 Node Position along wave with no motion / zero amplitude AS 8 Antinode Position along wave with maximum amplitude. AS 8 Constructive Interference Two waves' path difference is either λ or nλ, OR phase difference is 360°or n ×360° or n2π AS 8 Destructive Interference Two waves' path difference is either λ/2 or (n+ ½) λ OR phase difference is odd multiple of either 180° or π rad AS 8 Two waves of same frequency/wavelength travelling (along the same line) in opposite Stationary Waves directions overlap/meet. The resultant displacement is the sum of displacements of each wave / produces nodes and antinodes AS 8 Stationary Wave Does not transfer energy (no energy transfer). The amplitude of standing wave varies along Properties its length/nodes and antinodes.Neighboring points (in inter-nodal loop) vibrate in phase AS 8 Fringe The separation between one bright fringe & the next bright fringe. Width/Separation AS 8 Interference When two waves superpose/overlap, the resultant displacement is the sum of individual displacements of overlapping waves, forming alternating maxima and minima. AS 8 Diffraction When a wave (front) passes by/incident on an edge/slit, the wave spreads into the geometrical shadow AS 8 Diffraction Grating When waves pass through the gaps / slits in the grating, the wave bends/spreads (into the geometrical shadow) AS 8 Refraction The change in direction of a wave due to change in speed. AS 9 Ampere If a charge of 1 Coulomb passes through an electrical component per second, then the current maintained is 1 Ampere AS 9 Charge Charge = current x time AS 9 Coulomb The SI unit of electrical charge. A charge of 1C passes a point when a current of 1A flows for 1s. AS 9 Electric Current It is the amount of charge flowing pass a point per unit time.Or rate of flow of charged particles. AS 9 Ohm volt/ampere AS 9 Ohm’s Law The current through a metallic conductor is proportional to the P.D across it provided that its temperature remains constant. AS 9 Potential Difference Energy converted from electrical to other forms of energy per unit charge that passes through it. AS 9 Quantised Charge only exists in discrete amounts. Charge on carriers is quantised. AS 9 Resistance The ratio of P.D over the current for an electrical component AS 9 Resistivity The resistivity of a wire of a particular material is its resistance for unit length. AS 9 Thermistor A specific type of resistor, in which, as temperature increases, the magnitude of the resistor’s resistance decreases, & vice versa. AS 9 Volt P.D between two points in a circuit in which 1J of energy is converted when 1C of charge passes from one point to the other. AS 10 E.m.f & P.D While e.m.f refers to the amount of energy converted into electrical energy per unit charge supplied, P.D refers to the amount of electrical energy converted into other forms of energy per unit charge supplied. The e.m.f of a source is equal to the potential difference across its terminals as the current approaches zero. AS 10 Electromotive Force Energy converted from chemical into electrical energy per unit charge. AS 10 Internal Resistance (resistance of the cell) causing loss of voltage or energy loss in cell AS 10 Kirchhoff’s 1st Law The sum of currents into a junction = sum of currents out of junction, Because charge cannot be created or destroyed (conservation of charge). AS 10 Kirchhoff’s 2nd Law Sum of e.m.f.’s = sum of p.d.’s around a loop/circuit. Because any gains in electrical energy of a charge must be balanced by corresponding losses of energy (conservation of energy). AS 10 Law of Conservation of Charge Kirchhoff’s First & Second Laws are in correspondence & actually are an appreciation of the Law of Conservation of Charge & the Law of Conservation of Energy respectively. 10 Output Power (Circuit) A battery delivers the maximum power to a circuit when the load resistance of the circuit is equal to the internal resistance of the battery. When load resistance is zero, power dissipated by load is zero because P=I^2R. When load resistance is very large, power dissipated gets very small as the current through the load is reduced significantly. AS 10 Potentiometer When a potential divider arrangement is used to compare e.m.fs of two sources, it is known a potentiometer. AS 11 Alpha particle Either helium nucleus OR particle containing two protons and two neutrons with mass 4u. Radiation can be deflected in electric/magnetic fields, and absorbed by thin paper or few cm of air. Radiation is highly ionizing. Baryon A type of hadron particle that is made up of three quarks (e.g. proton and neutron). AS 11 Beta Particle Produced due to weak nuclear force/interaction. β-particles are fast moving electrons with a range of speeds up to 0.99c. This radiation can be deflected by electric and magnetic fields or negatively charged, and absorbed by few (1 – 4) mm of aluminum. 0.5 to 2m for range in air. AS 11 Gamma Radiation γ- radiation is part of the electromagnetic spectrum with wavelengths between 10^(-11) m and 10^(-13) m. Hadron Class of heavy particles made up of quarks held together by strong nuclear force. Hadron is not a fundamental particle. Isotopes Atoms (same element) which have the same proton number, but a different nucleon number / number of neutrons. Meson A type of hadron particle that is made up of two quarks. 11 11 AS 11 11 https://9702-physics.blogspot.com/p/definitions.html 3/8 4/5/23, 2:11 PM Cambridge A Level Physics: Definitions AS 11 Nucleon (mass) Number The number of protons together with the number of neutrons in the nucleus is called the nucleon number (or mass number) A AS 11 Proton Number The number of protons in the nucleus of an atom ( aka atomic number) Z The Atom The simple model of the atom is made up of three sub-atomic particles: The proton (which is positively charged), the neutron (which is uncharged but equal in mass to the proton), & the electron (which is negatively charged & equal to the charge on the proton, but much smaller in size & mass). AS 11 Jump to sections: [AS Chapters][A2 Chapters] =================== A2 Level Physics Terms & Concepts Useful set of flashcards for A2 revision https://quizlet.com/392317817/cie-physics-a-level-definitions-flash-cards/ Lvl Ch Term Definition A2 12 Angular displacement the angle through which the object has moved A2 12 Radian angle subtended at the centre of a circle by an arc of length equal to the radius of the circle A2 12 Angular velocity rate of change of angular displacement A2 12 Angular acceleration rate of change of angular velocity A2 13 Newton’s Law of any two point masses attract each other a force that is directly proportional to the product of Gravitation their masses and inversely proportional to the square of the separation A2 13 Gravitational field gravitational force exerted per unit mass on a small object placed at that point strength, g A2 13 Field of force A region of space where a force acts (on a particle of certain property) A2 13 Gravitational potential work done per unit mass in bringing unit mass from infinity to the point A2 13 Geostationary orbits Orbit in which a satellite is positioned so that it orbits the Earth the same rate as the Earth’s rotation. Satellite remains above a fixed point on the Earth’s surface A2 15 Mole amount of that substance which contains the same number of particles as there are in 0.012 kg of carbon-12 A2 15 Avogadro's constant amoung of carbon-12 atoms in 12g of Carbon-12 A2 15 Boyle’s law pressure exerted by a fixed mass of gas is inversely proportional to its volume, provided the temperature of gas remains constant A2 15 Charles’ Law temperature acting on a fixed mass of gas is directly proportional to its volume, provided the pressure of gas remains constant A2 15 Ideal gas Gas that behaves and obeys all law A2 14 Thermal Equilibrium a condition when two or more objects in contact have the same temperature so that there is no net flow of thermal energy A2 14 Thermocouple device consisting of wires of two different metals across which an emf is produced when the two junctions of the wires are at different temperature A2 14 Temperature Amount of average kinetic energy of particles, and shows direction of net heat flow between two bodies in contact. A2 14 Fixed points Standard reference temperatures that are used when calibrating thermometers. A2 14 Absolute zero Temperature at which atoms have minimum or zero energy. A2 14 Calibration Uses fixed points as upper/lower points and assumes a linear change of property with temperature. A2 14 Thermodynamic / Scale does not depend on the property of a substance Absolute Scale A2 16 Internal energy A2 16 First Law of the increase in internal energy of a body is equal to the thermal energy transferred to it by Thermodynamics heating plus the mechanical work done on it A2 14 Heat / Thermal Energy energy transferred from one object to another because of temperature difference. Increases internal energy. A2 14 Specific heat capacity energy required per unit mass of the substance per unit °C to raise the temperature by 1 K or 1°C. A2 14 Specific latent heat energy required per kilogram of the substance to change its state without any change in temperature A2 14 Specific latent heat of fusion energy required per kilogram of a substance to change it from solid to liquid without a change in temperature A2 14 Specific latent heat of vaporisation energy required per kilogram of a substance to change it from liquid to gas without a change in temperature A2 14 Boiling The process by which a liquid changes into its gaseous state at a constant specific temperature, known as boiling point. Heat energy goes towards overcoming intermolecular forces to move the atoms far enough so that interatomic forces and potential energy are negligible. A2 16 Brownian Motion The haphazard or random movement of tiny suspended particles (such as smoke, pollen etc.) in a fluid is known as Brownian Motion. sum of the random distribution of kinetic and potential energies of its atoms or molecules https://9702-physics.blogspot.com/p/definitions.html 4/8