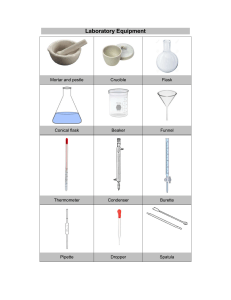
Aims and objectives : To observe the colors that appears as a result of electron transitions in metal ions. Apparatus: Bunsen burner Spatula 2x 100 ml beaker Petri dish Hydrochloric acid (32%) Pipette Procedure: 1. The plastic tube of the Bunsen burner was attached to the yellow gas outlet and switched. 2. Using a match, the match was light and adjusted the Bunsen burner to a blue flame. 3. The spatula was heated directly on the flame, washed with tap water, dried, then dipped in a beaker filled with 5 ml HCl and then placed back on the flame so that all the HCl dissolves. 4. In a second beaker, 2l of HCl was obtained. 5. Using a petri dish, obtain approximately 0.1g of the metal ions 6. Using a pipette, add 2 drops of HCl into the dish to saturate the salt. Then place spatula on it to collect the salt 7. Place spatula on flame and observe flame color 8. Repeat procedure for all metal ions Results: Discussion and conclusion Post-lab questions: a) (i) sodium has a (ii) lithium has a (iii) Barium has a (iv) Potassium has a (v) Calcium has a b) (i) (ii) (iii) c) Reference