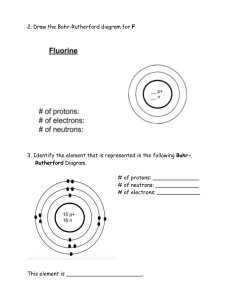
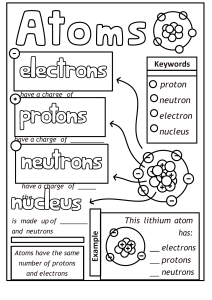
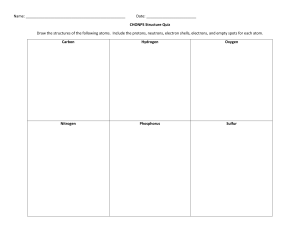
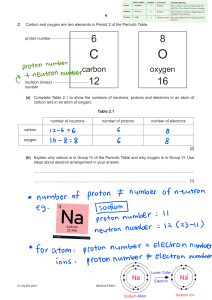
DAILY LESSON PLAN IN Chemistry (Physical Science) – GRADE 11 November 28, 2018 ROSA MIA P. MATANGGAN Demonstration Teacher I – OBJECTIVES A. Content Standard The learners demonstrate an understanding of: how the concept of the atom evolved from Ancient Greek to the present B. Performance Standard The learners shall be able to: make a scrapbook/atom handbook/bulletin board/digital infographic/interactive notebook about how the idea of the atom, along with the idea of the elements evolved. C. Learning Competency/Objective The learners describe the nuclear model of the atom and the location of its major components (protons, neutrons, and electrons) (S11/12PS-IIIb-10) II – CONTENT A. How the idea of the atom, along with the idea of the elements evolved (STRUCTURE OF THE ATOM) III – LEARNING RESOURCES A. References: PHYSICAL SCIENCE (CHED in collaboration with PNU) K to 12 Basic Education Curriculum, Senior High School-Core Subject, p. 2 https://www.youtube.com/watch?v=o-3I1JGW-Ck https://www.youtube.com/watch?v=R1RMV5qhwyE https://www.youtube.com/watch?v=BvYiMnRN__A B. Other reference: PhET Simulation C. Science concepts: Atoms can be visualized as a spherical particle with tiny, positively charged center (atomic nucleus) that contains most of the mass; diffuse outer layer that is negatively charged. Atomic nucleus is tiny, positively charged, massive center of an atom that contains protons and neutrons; contains most of the mass of an atom Electrons are found outside the nucleus of an atom moving around it with specific energy levels. Protons and electrons are the only particles that have a charge. Protons and neutrons have essentially the same mass. The mass of an electron is so small we ignore it. Each atom contains the same number of protons and electrons. The atomic number tells you both the number of protons and electrons in an atom. All atoms of a given element have the same number of protons in the nucleus. Atomic Number (Z) = number of protons. In a neutral atom, the # of protons = the # of electrons Atomic Mass Number - equal to the number of protons plus neutrons. + At. Mass = p IV – PROCEDURES A. Pre- Activities a. Management of Learning (MOL) + n Prayer Energizer House Rules and Regulations b. Review – “4 PICS 1 WORD!” Teacher divides the class into ten (10) groups in which each group is composed of both male and female students. Distribute to each group 5 strips of empty papers. Let them play the game 4 PICS 1 WORD. They will be instructed that their points for all activities will be consolidated at the end. The more points they have the better grade they’ll get. After the activity, students will complete the timeline for the atomic theory/model from Greeks to the most modern version of the atom. Pose the following questions: 1. Why the idea of atom that is indivisible didn’t work all throughout the timeline? 2. Why Neils Bohr’s idea about electron’s orbit didn’t work through the end? c. Motivation: “Picture analysis” Teacher shows two (2) pictures and pose the following questions: 1. Which is really an Atom? 2. Which do you think is the correct model of an atom? Teacher establishes purpose of the activity and relates it to the present topic that they are going to tackle for the session. d. Pre- Assessment: ANTICIPATION-REACTION GUIDE (ARG) Each group will answer the anticipation reaction guide. They are given 10 strips of empty papers. The teacher will instruct them to fold the strip of paper into half. The left half will be for their anticipation column and the remaining half is for their reaction column. Let them know that the other half of the strip of paper will be answered after all the discussions were made. Directions: Decide whether the statement is true or false. Write your answer in the anticipation column of your table while leave the reaction column as blank. (Note: reaction column should be answered after the discussion of concepts) ANTICIPATION STATEMENT 1. Protons and electrons are found in the nucleus of an atom. 2. Electron is a negatively-charged particle found inside the atom. 3. Each atom contains the same number of protons, neutrons and electrons. 4. Atomic Mass Number is equal to the number of protons plus neutrons. 5. Protons have a positive charge (+). 6. Electrons move around the nucleus of an atom. 7. An atom can have the same number of protons but different number of neutrons. 8. Neutron has no charge. 9. The atomic number tells you both the number of protons and electrons in an atom. 186Re 10. has 75 protons, 111 neutrons and 75 75 electrons. REACTION B. Activities 1. Activity 1: (Developmental activity)“My atomic model” Teacher instructs the group to make their own atomic model incorporating/reconciling all the ideas about atom from the timeline presented in the previous activity. They will use indigenous materials such as seeds (3 types) and sticks/stalks to make a model. Rubrics will be presented by the teacher prior to the activity. Students will describe their model in the class after 5 minutes using English language. 2. Activity 2: “Let’s simulate!” Students will be grouped into five (5). Each group is assigned specific atom/element to be simulated. The group should assign members who will act as protons, neutrons or electrons and emphasize the location of each of them in an atom. Determining the correct number of protons, neutrons and electrons in each atom must be taken into considerations too. Group 1: Boron group Group 2: Carbon group Group 3: Nitrogen group Group 4: Oxygen group Group 5: Fluorine group After 5 minutes preparation, each group will present their simulation and let the other group guess what element/atom is being simulated through counting their number of protons. Then, ask volunteers from the group to describe the atom presented in terms of how many protons, neutrons, and electrons does it have and its location in the atom. The more groups who can guess the correct answer the additional points (10 pts.) will be given to the performers. D. Analysis: For Activity 1: Teacher poses 1. 2. 3. 4. 5. 6. the following questions: What represents the seeds? How many protons does your model have? How many neutrons does your model have? What represents the sticks/stalks? How many electrons does your model have? Identify what atom is represented by your model based on its number of protons, neutrons and electrons. 7. Describe the structure of atoms you have including the locations of protons, neutrons and electrons. For Activity 2: Teacher poses the following questions: (Based on simulation) 1. Based on the simulation, identify what element/atom is being presented by each group? 2. How did the group represent their protons? Electrons? And neutrons? 3. How many protons, neutrons and electrons in the atom presented? E. Abstraction: Teacher selects any one, two, or all of the activities to generalize the structure of the atom. In doing this, students will perform activity (quiz bowl type from PhET simulation) o Video Clip: 3-5 minutes. Teacher shows related video/s about atom. Teacher asks: What is the importance of studying atoms in our life? F. Application: “Think-Pair-Share” V. EVALUATION Students will answer the ANTICIPATION-REACTION GUIDE specifically the reaction column. ANTICIPATION STATEMENT 1. Protons and electrons are found in the nucleus of an atom. 2. Electron is a negatively-charged particle found inside the atom. 3. Each atom contains the same number of protons, neutrons and electrons. 4. Atomic Mass Number is equal to the number of protons plus neutrons. 5. Protons have a positive charge (+). 6. Electrons move around the nucleus of an atom. 7. An atom can have the same number of protons but different number of neutrons. 8. Neutron has no charge. 9. The atomic number tells you both the number of protons and electrons in an atom. 186 Re 10. 75 has 75 protons, 111 neutrons and 75 electrons. REACTION VI. ASSIGNMENT Rubrics will be used for rating Prepared by: ROSA MIA P. MATANGGAN SHS Teacher II Activity Sheet Activity 1 “My Atomic Model” Objectives: Students will participate in an activity to make an atomic model showing the location of protons, neutrons and electrons. MATERIALS: Seeds (three different kinds) Modern Periodic Table of Elements Cartolina Sticks/stalks Glue/scotch tape Procedures: 1. Based on the timeline the teacher presented in the review portion, construct your own atomic model incorporating/reconciling all the ideas about atoms. 2. Gather the materials needed. Decide how many protons, neutrons and electrons you will need for your model. Seeds (3 different kinds) will represent your protons, neutrons and electrons; sticks/stalks will represent for the electrons’ orbit or electron cloud. 3. Build the Nucleus ((1 seed type= protons; another seed type =neutrons).). The nucleus would look something like this: 4. Place the Electrons. 3rd seed type with stick/stalk. The electrons are found outside the nucleus. How you place them depends on which model of atomic structure your group had decided and had understood. Use sticks/stalks to represent your electron’s orbit. This may look either of this: 5. After 5 minutes, present your model in the class using English language. 6. Rubrics will be used for rating. Analysis: 1. What represents the seeds? 2. How many protons does your model have? 3. How many neutrons does your model have? 4. What represents the sticks/stalks? 5. How many electrons does your model have? 6. Identify what atom is represented by your model based on its number of protons, neutrons and electrons. 7. Describe the structure of atoms you have including the locations of protons, neutrons and electrons. Performance Task “Let’s Simulate” Objectives: Students will participate in an activity to simulate specific atom and describe the location of protons, neutrons and electrons inside the atom. Determine the number of protons, neutrons and electrons. MATERIALS: Activity sheets Modern Periodic Table of Elements Colored papers Procedures: 1. Find your group. 2. You will be given specific atom/element to simulate with. Be sure that the other group will not know about your assigned atom. 3. You are given colored papers to be used in representing protons, neutrons and electrons. Decide whether what color you will use in protons, neutrons, as well as electrons. 4. Based on your assigned atom, determine your number of protons, neutrons and electrons. 5. Leader will select member who will represent as protons, neutrons and electrons of the element which is assigned to the group. The member who will be assigned as proton should hold the colored paper which the group had decided; same with the electrons and neutrons. 6. After 5-minute preparation. The group will do the simulation emphasizing how each part of the atom behaved. 7. While the group is doing the simulation, the other groups in their seats will guess what atom has been simulated and determine its number of protons, neutrons and electrons through answering the table below: Group # Atom # of protons # of neutrons # of electrons Atomic mass 1 2 3 4 5 8. When more groups are able to guess the atom being simulated, additional 10 points will be given to the group performers. 9. Point system will still be used. Analysis: 1. Based on the simulation, identify what element/atom is being presented by each group? 2. How did the group represent their protons? Electrons? And neutrons? 3. How many protons, neutrons and electrons in the atom presented? ATOM MODEL GRADING RUBRIC Atom model Correct number of protons & placement of protons in the model: ________________ (2) Correct number of neutrons & placement of neutrons in the model: ______________ (2) Correct number of electrons & placement of electrons in the model: ______________ (2) Respective sizes of protons, neutrons, and electrons is correct: _________________ (3) (i.e. protons and neutrons are the same size electrons are smaller) Key Contains the group name: ___________________________________________________ (2) Contains the atoms name:___________________________________________________ (2) Correctly identifies the protons, neutrons, and electrons ________________________ Correctly tells how many electrons are in each of the shells (3) ______________________ (4) Overall Construction & Neatness Did not do (0) Timeliness: 5th (2) Poor Average (4) (6) 4th (4) 3rd (6) Above Average Outstanding ____________ (10) (8) 2nd (8) (10) 1st (10) ______________(10) Total Points __________ __ (40) --------------------------------------------------------------------------------------------------------------------------------------------------------------------------- ATOM MODEL GRADING RUBRIC Atom model Correct number of protons & placement of protons in the model: ________________ (2) Correct number of neutrons & placement of neutrons in the model: ______________ (2) Correct number of electrons & placement of electrons in the model: ______________ (2) Respective sizes of protons, neutrons, and electrons is correct: _________________ (3) (i.e. protons and neutrons are the same size electrons are smaller) Key Contains the group name: ___________________________________________________ (2) Contains the atoms name:___________________________________________________ (2) Correctly identifies the protons, neutrons, and electrons ________________________ Correctly tells how many electrons are in each of the shells (3) ______________________ (4) Overall Construction & Neatness Did not do (0) Timeliness: 5th (2) Total Points Poor Average (4) (6) 4th (4) 3rd (6) Above Average Outstanding ____________ (10) (8) 2nd (8) (10) 1st (10) ______________(10) __________ __ (40)