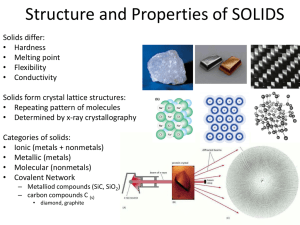
Lab Investigation: Bonding in Solids Dhimahi Raval Purpose: This lab aims to use physical properties to classify the type of solid for eight common substances. Introduction: There are four types of solids covered in this lab: Ionic crystals, Metallic crystals, Molecular crystals, and Covalent crystals. Ionic crystals are formed from the reaction between metal and non-metal ions. A metal and a non-mental come together in this case to create oppositely charged ions that are electrostatically attracted. This concludes that electrostatic forces hold the ionic crystals together. The ions are arranged in a crystal lattice structure with oppositely packed positive and negative ions. Ionic crystals are usually brittle and rigid and difficult to distinguish because of the tightly packed positive and negative ions. They also have a very high melting and boiling point because of the tight packaging. Ionic crystal solids do not conduct electricity but the solution they are dissolved in does conduct electricity. Metallic crystals are composed of closely packed atoms held together by electrostatic interactions and free-moving electrons. Electron sea theory states that electrons in a metallic crystal have the ability to move freely around the positively charged nuclei and can be used to explain the structure of metallic crystals where mobile electrons are shared over many nuclei. The ability of metallic crystals to have chemical bonds with electrons of these properties allows them to be good conductors of heat and electricity. They are also shiny and malleable in appearance because of the “electron sea” which allows them to slide over each other. Molecular crystals are substances that form a crystal lattice when in solid form. Intermolecular forces such as London-dispersion forces, Dipole-dipole forces, and Hydrogen bonding are used to hold these structures together. These intermolecular forces are weaker than the intramolecular forces which are why molecular crystals tend to have lower melting points and will be less hard than ionic crystals. The ability of molecular crystals to only contain neutral molecules prohibits them from conducting electricity. Covalent network crystals are formed by the covalent bonds participating in an interwoven structure. These crystals are generally bonded together into a three-dimensional network o layers of two-dimensional networks. The covalent bonds in these crystals have a large amount of strength and a large amount of energy is required to break the bonds and melt in this network resulting in high melting points. The covalent network crystals tend to have extreme hardness due to the three-dimensional crystal structure. The electrons in these crystals are not mobile or free-moving as a result not allowing the solids to be good conductors of electricity. Materials: ❖ PPE: Goggles ❖ PPE: Gloves ❖ Stirring Rod ❖ Scoopula ❖ Spot Plate ❖ Deionized Water ❖ Distilled Water ❖ Paper towel ❖ Conductivity apparatus ❖ Pippete ❖ Aluminum ❖ Zinc Sulfate ❖ Tin ❖ Citric Acid ❖ Sulfur ❖ Dextrose ❖ Activated charcoal ❖ Tin (II) Chloride Procedure: Refer to the “Lab Investigation: Bonding in Solids” handout for all the procedures performed during the lab. Observations: Zinc Tin Sulfate Texture Melting points Electrical conductivity Dextrose Citric Acid Charcoal Tin (II) Aluminum Chloride Hard, Black, Yellow, Grainy, Sugar-like Crystal-like, Spherical, white, smooth, soft, opaque, white, grains, shiny, dense, really translucent, opaque, chalky, easily hard particles, small big chunks crushed light opaque easily Solubility Sulfur Brittle, slightly Odour Activated shiny, reflects powder, fluffy, powder, crushed opaque, small slightly brittle, hard, hard, shiny, translucent Subtly Metallic Very light Sour Non-acidic Sour/tart acidic smell soft smell smell soft smell smell Soluble Insoluble Insoluble Insoluble Slightly Soluble Soluble Insoluble 680° 232° 3550° 113° 146° 153° 247° 660° 0.07 A 5.85 A 0A 0A 0A 0.01 A 0.05 A 6.20 A soluble Odorless Sweet metallic smell Conclusions: Zinc Sulfate - Ionic Crystal Zinc sulfate is hard, brittle, soluble and it also showed some conductivity with a high melting point which all falls in the criteria of substances considered ionic crystal solids. Thus, zinc sulfate is an ionic crystal solid. Tin - Metallic Crystal The properties of tin such as hard, shiny, insoluble, with a moderate melting point of 232° and electric conductivity classifies it as a solid metallic crystal as metallic crystals tend to have the same properties as this solid. Activated Charcoal - Covalent Network Crystal The ability of activated charcoal to not dissolve and have no conductivity all the while having an extremely high melting point, differentiates it from the ionic crystals and classifies it as a covalent network crystal with covalent bonds. Sulfur - Molecular Crystal The property of sulfur to have a very low melting point, to be insoluble in water, and to have no conductivity makes it fall in the category of molecular crystals because molecular crystals have neutral molecules which cannot pass electricity and the low melting point would be the result of IMFs in the solid. Dextrose - Molecular Crystal Dextrose is a molecular crystal because of its slight solubility in water and no conductivity with a low melting point similar to the substance that was tested out in the lab. IMFs would be the cause of these physical properties of the molecular crystal solid. Citric Acid - Ionic Crystal Citric Acid has the texture properties of hard, brittle, and slightly soluble in water which puts it in the category of an ionic crystal. Citric acid’s ability to conduct electricity prohibits it from becoming a molecular or covalent network crystal and its positive acceptance of water solubility prohibits it from becoming a metallic crystal. Thus, citric acid is an ionic crystal. Tin (II) chloride - Ionic Crystal Tin (II) chloride is an ionic crystal because it has both the metal and non-metal needed to react with each other. It also has the physical characteristics of brittle and hard which are ionic crystal properties. Thus, this substance is an ionic crystal. Aluminum - Metallic Crystal Aluminum is a metallic crystal as it is extremely malleable due to the “electron sea” possessing one of the main properties of a metallic crystal. Aluminum is also insoluble in water and a good conductor of electricity fulfilling all the criteria of a metallic crystal. Thus, aluminum is a metallic crystal due to its mobile electrons over a number of positive nuclei. Errors: ● One of the possible errors in this lab conducted can be a contaminated spot plate or lab equipment which might react with the experimental substances and might give a false electric conductivity reading. ● Another error can be contaminated distilled water in use during the lab. Due to the large number of people conducting the experiment, the distilled water might have a high chance of contamination and getting other ions dissolved in it due to the experimental substances and lab equipment which might interfere with our chemical test and give a false reading for solubility. Other Physical properties which can be used to classify solids: ● Acid Reaction - Experimenting with the solids with acids gives us an idea of the intermolecular forces, ph value, and bonds present in solids which can be used to classify the type of solid. ● Magnetism - Solids can be classified into diamagnetic, paramagnetic, ferromagnetic, and many more which is one of the main physical properties used to determine the type of solid. Food Label: (Pringles) Wheat Starch (C45H80O70) - Wheat starch has a flour-like odor, flavor, and appearance. It is powdery and with very minimal viscosity. It is insoluble in water and cannot conduct electricity. It has a high melting point and can be considered a covalent network crystal because of all of its properties. Maltodextrin (C6H12O6) - Maltodextrin has a texture of white powder and softness. It is soluble in water and it cannot conduct electricity. It has high boiling and melting points. Thus, it can be considered a molecular crystal solid. Cornstarch (C12H22O11) - Cornstarch has a medium melting point and it does not conduct electricity. Cornstarch is a powdery substance similar to wheat starch. It is insoluble in water and thus it can be considered a covalent network crystal solid. Diglycerides (C6H8O6) - Diglycerides have a low melting point. It is insoluble in water and is an insulator of electricity. It is formed due to the fatty acid chains and it can be considered a molecular crystal due to its properties. References DiGiuseppe, M. Haberer, S., Salciccioli K., Sanader, M., & vavistas, A. (2012). Chemistry 12: University preparation. Toronto, ON: Nelson Metallic Crystals. Boundless. (n.d.). Retrieved March 10, 2023, from https://bluebox.creighton.edu/demo/modules/en-boundless-old/www.boundless.com /chemistry/textbooks/boundless-chemistry-textbook/liquids-and-solids-11/types-of. Solids - definition, types, and classification. Unacademy. (2022, April 24). Retrieved March 11, 2023, from https://unacademy.com/content/neet-ug/study-material/physics/solids-definition-ty pes-and-classification/ U.S. National Library of Medicine. (n.d.). Citric acid. National Center for Biotechnology Information. PubChem Compound Database. Retrieved March 11, 2023, from https://pubchem.ncbi.nlm.nih.gov/compound/Citric-Acid#section=Color-Form Using characteristics of minerals to identify them. Illinois State Geological Survey. (n.d.). Retrieved March 11, 2023, from https://isgs.illinois.edu/outreach/geology-resources/using-characteristics-mineralsidentify-them#:~:text=Most%20minerals%20can%20be%20characterized,cleavage%2 C%20fracture%2C%20and%20tenacity.