Antibiotic Guidelines
2015-2016
Treatment Recommendations
For Adult Inpatients
Also available online at
insidehopkinsmedicine.0rg/amp
Table of contents
1. Introduction ............................................................................................ 3
2. Johns Hopkins Hospital formulary and restriction status .................... 6
2.1 Obtaining ID approval ........................................................................6
2.2 Formulary .........................................................................................7
3. Agent-specific guidelines ...................................................................... 8
3.1 Antibiotics ........................................................................................8
Ceftaroline ......................................................................................8
Ceftolozane/tazobactam .................................................................8
Colistin ...........................................................................................9
Daptomycin ................................................................................. 10
Ertapenem................................................................................... 11
Fosfomycin .................................................................................. 11
Linezolid ...................................................................................... 12
Tigecycline .................................................................................. 13
Trimethoprim/sulfamethoxazole ................................................... 14
3.2 Antifungals..................................................................................... 16
AmBisome® ................................................................................ 16
Micafungin ................................................................................... 17
Posaconazole .............................................................................. 18
Voriconazole ................................................................................ 19
Azole drug interactions................................................................. 20
3.3 Vaccines ....................................................................................... 23
Pneumococcal vaccines ............................................................... 23
4. Organism-specific guidelines .............................................................. 24
4.1 Anaerobes..................................................................................... 24
4.2 Propionibacterium acnes................................................................ 25
4.3 Streptococci.................................................................................. 27
4.4 Multi-drug resistant Gram-negative rods .......................................... 28
5. Microbiology information .................................................................... 31
5.1 Interpreting the microbiology report................................................ 31
5.2 Spectrum of antibiotic activity......................................................... 32
5.3 Interpretation of rapid diagnostic tests ............................................ 34
5.4 Johns Hopkins Hospital antibiogram ............................................... 36
6. Guidelines for the treatment of various infections...........................39
6.1 Abdominal infections .............................................................39
Biliary tract infections ................................................................... 39
Diverticulitis ................................................................................. 40
Pancreatitis ................................................................................. 41
Peritonitis (including SBP, GI perforation and peritonitis
related to peritoneal dialysis) ........................................................ 42
6.2 Clostridium difficile infection (CDI) ............................................ 47
6.3 Infectious diarrhea ..................................................................... 51
6.4 H. pylori infection ....................................................................... 54
6.5 Gynecologic and sexually transmitted infections ..................... 56
Pelvic inflamatory disease ............................................................ 56
Endomyometritis .......................................................................... 56
Bacterial vaginosis ....................................................................... 57
Trichomoniasis ............................................................................ 57
Uncomplicated gonococcal urethritis, cervicitis, proctitis ............... 57
Syphilis........................................................................................ 58
6.6 Catheter-related bloodstream infections .................................. 60
(continued on next page)
1
Table of contents
6.7 Endocarditis ................................................................................ 65
6.8 Pacemaker/ICD infections......................................................... 71
6.9 Central nervous system (CNS) infections ................................. 73
Meningitis .................................................................................... 73
Encephalitis ................................................................................. 75
Brain abscess .............................................................................. 76
CNS shunt infection...................................................................... 76
Antimicrobial doses for CNS infections.......................................... 77
6.10 Acute bacterial rhinosinusitis (ABRS) .....................................78
6.11 Orbital cellulitis .....................................................................80
6.12 Pulmonary infections.................................................................. 82
COPD exacerbations .................................................................... 82
Community-acquired pneumonia ................................................... 83
Healthcare-acquired pneumonia. ................................................... 87
Ventilator-associated pneumonia ................................................... 88
Cystic fibrosis .............................................................................. 91
6.13 Respiratory virus diagnosis and management ......................... 93
6.14 Tuberculosis (TB) ........................................................................ 95
6.15 Sepsis with no clear source ....................................................... 99
6.16 Skin, soft-tissue, and bone infections......................................100
Cellulitis ..................................................................................... 100
Cutaneous abscess.................................................................... 101
Management of recurrent MRSA infections .................................. 102
Diabetic foot infections ............................................................... 103
Surgical-site infections................................................................ 105
Serious, deep soft-tissue infections (necrotizing fasciitis).............. 107
Vertebral osteomyelitis, diskitis, epidural abscess ....................... 108
6.17 Urinary tract infections (UTI)....................................................110
Bacterial UTI (including pyelonephritis and urosepsis) ................... 110
6.18 Candidiasis in the non-neutropenic patient ............................115
6.19 Guidelines for the use of prophylactic antimicrobials .................121
Pre-operative and pre-procedure antibiotic prophylaxis................. 121
Prophylaxis against bacterial endocarditis .................................. 125
Prophylactic antimicrobials for patients with
solid organ transplants ............................................................... 126
6.20 Guidelines for the use of antimicrobials in
neutropenic hosts. ....................................................................129
Treatment of neutropenic fever................................................... 129
Prophylactic antimicrobials for patients with
expected prolonged neutropenia ................................................ 131
Use of antifungal agents in hematologic
malignancy patients ............................................................. 133
7. Informational guidelines .................................................................137
7.1 Approach to the patient with a history of penicillin allergy ................ 137
8. Infection control ..............................................................................139
8.1 Hospital Epidemiology & Infection Control .................................... 139
8.2 Infection control precautions ....................................................... 141
8.3 Disease-specific infection control recommendations ..................... 142
10. Appendix:
A. Aminoglycoside dosing and therapeutic monitoring ........................ 145
B. Vancomycin dosing and therapeutic monitoring.............................. 150
C. Antimicrobial therapy monitoring ................................................... 153
D. Oral antimicrobial use ................................................................... 154
E. Antimicrobial dosing in renal insufficiency ....................................... 155
F. Cost of select antimicrobial agents ................................................ 159
2
1. Introduction
Introduction
Antibiotic resistance is now a major issue confronting healthcare
providers and their patients. Changing antibiotic resistance patterns,
rising antibiotic costs and the introduction of new antibiotics have
made selecting optimal antibiotic regimens more difficult now than
ever before. Furthermore, history has taught us that if we do not
use antibiotics carefully, they will lose their efficacy. As a response
to these challenges, the Johns Hopkins Antimicrobial Stewardship
Program was created in July 2001. Headed by an Infectious Disease
physician (Sara Cosgrove, M.D., M.S.) and an Infectious Disease
pharmacist (Edina Avdic, Pharm.D., M.B.A), the mission of the
program is to ensure that every patient at Hopkins on antibiotics
gets optimal therapy. These guidelines are a step in that direction.
The guidelines were initially developed by Arjun Srinivasan, M.D., and
Alpa Patel, Pharm.D., in 2002 and have been revised and expanded
annually.
These guidelines are based on current literature reviews, including
national guidelines and consensus statements, current microbiologic
data from the Hopkins lab, and Hopkins’ faculty expert opinion.
Faculty from various departments have reviewed and approved these
guidelines. As you will see, in addition to antibiotic recommendations,
the guidelines also contain information about diagnosis and other
useful management tips.
As the name implies, these are only guidelines, and we anticipate
that occasionally, departures from them will be necessary. When these
cases arise, we will be interested in knowing why the departure is
necessary. We want to learn about new approaches and new data as
they become available so that we may update the guidelines as needed.
You should also document the reasons for the departure in the patient’s
chart.
Sara E. Cosgrove, M.D., M.S.
Director, Antimicrobial Stewardship Program
Edina Avdic, Pharm.D., M.B.A
ID Pharmacist
Associate Director, Antimicrobial Stewardship Program
Kate Dzintars, Pharm.D.
ID Pharmacist
Janessa Smith, Pharm.D.
ID Pharmacist
3
1. Introduction
The following people served as section/topic reviewers
N. Franklin Adkinson, M.D. (Allergy/Immunology)
Paul Auwaerter, M.D. (Infectious Diseases)
Robin Avery, M.D. (Infectious Diseases)
John Bartlett, M.D. (Infectious Diseases)
Dina Benani, Pharm. D. (Pharmacy)
Michael Boyle, M.D. (Pulmonary)
Roy Brower, M.D. (Critical Care and Pulmonary)
Karen Carroll, M.D. (Pathology/Infectious Diseases)
Michael Choi, M.D. (Nephrology)
John Clarke, M.D. (Gastroenterology)
Todd Dorman, M.D. (Critical Care)
Christine Durand, M.D. (Infectious Diseases)
Khalil Ghanem, M.D. (Infectious Diseases)
James Hamilton, M.D. (Gastroenterology)
Carolyn Kramer, M.D. (Medicine)
Pam Lipsett, M.D. (Surgery and Critical Care)
Colin Massey, M.D. (Medicine)
Lisa Maragakis, M.D. (Infectious Diseases)
Kieren Marr, M.D. (Infectious Diseases)
Robin McKenzie, M.D. (Infectious Diseases)
Michael Melia, M.D. (Infectious Diseases)
George Nelson, M.D. (Infectious Diseases)
Eric Nuermberger, M.D. (Infectious Diseases)
Trish Perl, M.D., M.Sc. (Infectious Diseases)
Stuart Ray, M.D. (Infectious Diseases)
Anne Rompalo, M.D. (Infectious Diseases)
Annette Rowden, Pharm.D. (Pharmacy)
Paul Scheel, M.D. (Nephrology)
Cynthia Sears, M.D. (Infectious Diseases)
Maunank Shah, M.D. (Infectious Diseases)
Tiffeny Smith, Pharm.D. (Pharmacy)
Jennifer Townsend, M.D. (Infectious Diseases)
Robert Wise, M.D. (Pulmonary)
Frank Witter, M.D. (OB-GYN)
How to use this guide
UÊ >V ÊÃiVÌÊLi}ÃÊLÞÊ}Û}ÊÀiVi`>ÌÃÊvÀÊÌ iÊV ViÊ>`Ê
dose of antibiotics for the particular infection.
UÊALL DOSES IN THE TEXT ARE FOR ADULTS WITH NORMAL
RENAL AND HEPATIC FUNCTION.
UÊÊvÊÞÕÀÊ«>ÌiÌÊ`iÃÊ "/Ê >ÛiÊÀ>ÊÀi>ÊÀÊ i«>ÌVÊvÕVÌ]Ê
please refer to the sections on antibiotic dosing to determine the
correct dose.
UÊÊÜ}ÊÌ iÊ>ÌLÌVÊÀiVi`>ÌÃ]ÊÜiÊ >ÛiÊÌÀi`ÊÌÊVÕ`iÊ
some important treatment notes that explain a bit about WHY the
particular antibiotics were chosen and that provide some important
tips on diagnosis and management. PLEASE glance at these notes
4
Contacting us
UÊÌLÌVÊ>««ÀÛ>\Ê1ÃiÊ* ÆÊÃi>ÀV ʺ>ÌLÌV]»ÊÌ iÊÃiiVÌÊ
ºÌLÌVÊ««ÀÛ>Ê*>}iÀ»
UÊÊ*i>ÃiÊ`ÊÌÊÃi`ÊÕiÀVÊ«>}iÃ
UÊÊ*i>ÃiÊV«iÌiÊÌ iÊvÀÊ>ÃÊ>VVÕÀ>ÌiÞÊ>ÃÊ«ÃÃLi°
UÊÊÊÀ`iÀÃÊvÀÊÀiÃÌÀVÌi`Ê>ÌLÌVÃÊ1-/ÊLiÊ>««ÀÛi`ÊÕiÃÃÊ
they are part of an approved order.
UÊÊ*i>ÃiÊÃiiÊ«>}iÊÈÊvÀÊÀiÊvÀ>ÌÊ>LÕÌÊLÌ>}Ê>««ÀÛ>°
UÊÌVÀL>Ê-ÌiÜ>À`Ã «Ê*À}À>\ÊÇ{xÇä
UÊviVÌÕÃÊ Ãi>ÃiÃÊ ÃÕÌÃ\ÊÎnäÓÈ
UÊ ÀÌV>Ê >ÀiÊ>`Ê-ÕÀ}iÀÞÊ* >À>VÞÊ­<>Þi`ÊΣӣ®\ÊxÈxäx
UÊ`ÕÌÊ«>ÌiÌÊ* >À>VÞÊ­<>Þi`ÊÇäää®\ÊxÈ£xä
UÊ7iLiÀ}Ê« >À>VÞ\Êxnn
UÊ >ÞÛiÜÊ«>ÌiÌÊ* >À>VÞ\Êääxn
UÊVÀL}ÞÊ>L\ÊxÈx£ä
A word from our lawyers
The recommendations given in this guide are meant to serve as
treatment guidelines. They should NOT supplant clinical judgment or
Infectious Diseases consultation when indicated. The recommendations
were developed for use at The Johns Hopkins Hospital and thus may
not be appropriate for other settings. We have attempted to verify
that all information is correct but because of ongoing research, things
may change. If there is any doubt, please verify the information in the
}Õ`iÊLÞÊV>}ÊÌ iÊ>ÌLÌVÃÊ«>}iÀÊÕÃ}Ê* Ê­Ãi>ÀV ʺ>ÌLÌV»®ÊÀÊ
Infectious Diseases.
Also, please note that these guidelines contain cost information
that is confidential. Copies of the book should not be distributed
outside of the institution without permission.
5
1. Introduction
when you are treating infections, as we think the information will prove
helpful. All references are on file in the office of the Antimicrobial
Stewardship Program (7-4570).
2.1 Obtaining ID approval
Obtaining ID approval
The use of restricted and non-formulary antimicrobials requires preapproval from Infectious Diseases. This approval can be obtained by any
of the following methods.
Approval method
* \ʺ>ÌLÌV»Ê
Overnight Approval
Ê
Ordersets (e.g. neutropenic
fever, etc.)
6
Notes
Ê/ iÊ«>}iÀÊÃÊ>ÃÜiÀi`ÊLiÌÜiiÊnÊ>°°Ê
and 10 p.m. PING the ID consult pager
if you fail to get a response from the ID
approval pager within 10 minutes.
Restricted antibiotics ordered between
10 p.m. and 8 a.m. must be approved
by noon the following morning.
UÊÊ*i>ÃiÊÀiiLiÀÊÌÊÃ}ÊÕÌÊÌ iÊii`Ê
for approval if you go off shift before
8 a.m.
These forms are P&T-approved for
specific agents and specific indications.
The following list applies to ALL adult floors and includes the status of
both oral and injectable dosage forms, unless otherwise noted.
Unrestricted
Amoxicillin
Amoxicillin/clavulanate
Ampicillin/sulbactam
(Unasyn®)
Ampicillin IV
Azithromycin
Cefazolin
Cefdinir
Cefotetan
Cefpodoxime
Ceftriaxone
Cefuroxime IV
Cephalexin
Clarithromycin
Clindamycin
Dicloxacillin
Doxycycline
Ertapenem
Erythromycin
Gentamicin
Metronidazole
Minocycline
Nitrofurantoin
Oxacillin
Penicillin V/G
Ribavirin oral
Rifampin
Streptomycin
Tobramycin
Trimethoprim/
sulfamethoxazole
Amphotericin B
deoxycholate
(Fungizone®)
Flucytosine
Itraconazole oral solution
Restricted (requires ID
approval)
Amikacin
Aztreonam
Cefepime
Ceftaroline1
Ceftazidime
Ceftolozane/tazobactam1
Ciprofloxacin
Colistin IV
Cytomegalovirus Immune
Globulin (Cytogam®)2
Daptomycin1
Fosfomycin3
Linezolid
Meropenem
Moxifloxacin
Nitazoxanide4
Palivizumab (Synagis®)5
Piperacillin/tazobactam
­<ÃÞ®)
Quinupristin/
dalfopristin (Synercid®)
Ribavirin inhaled5
Telavancin1
Tigecycline
Vancomycin
Liposomal amphotericin B
(AmBisome®)
Micafungin
Fluconazole6
Posaconazole
Voriconazole
1Approval must be obtained from Antimicrobial Stewardship Program 24h/7 days a week
2Approval required, except for solid organ transplant patients
3Approval must be obtained 24h/7 days a week
4Approval must be obtained from Polk Service or ID Consult
5Approval must be obtained from ID attending physician 24h/7 days a week
6Oral Fluconazole, when used as a single-dose treatment for vulvovaginal candidiasis or when
used in compliance with the SICU/WICU protocol, does not require ID approval
Restricted antimicrobials that are ordered as part of a P&T-approved critical pathway or order
set do NOT require ID approval.
REMINDER: the use of non-formulary antimicrobials is strongly discouraged. ID
approval MUST be obtained for ALL non-formulary antimicrobials.
NOTE: Formulary antivirals (e.g. Acyclovir, Ganciclovir) do NOT require ID approval.
7
2.2 Antimicrobial formulary and restriction status
Selected formulary antimicrobials
and restriction status
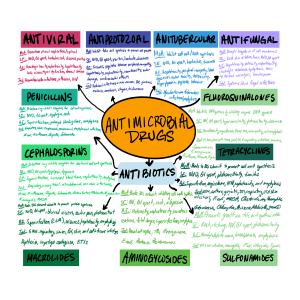
3.1 Agent-specific guidelines: Antibiotics
Antibiotics
Ceftaroline
Ceftaroline is a cephalosporin with in vitro activity against staphylococci
(including MRSA), most streptococci, and many Gram-negative bacteria.
It does NOT have activity against Pseudomonas spp. or Acinetobacter
spp. or Gram negative anaerobes.
Acceptable uses (Cases must be discussed with Infectious Diseases
and Antimicrobial Stewardship Program)
UÊÊ-iiVÌÊV>ÃiÃÊvÊ,-Ê«iÕ>ÊÀÊÌ iÀÊÃiÛiÀiÊviVÌÃÊÜ iÊ
Gram negative coverage is also needed
UÊÊ >VÌiÀi>ÊÀÊi`V>À`ÌÃÊV>ÕÃi`ÊLÞÊ,-ÊÊ>Ê«>ÌiÌÊv>}Ê
6>VÞVÊÌ iÀ>«ÞÊ>ÃÊ`iwi`ÊLÞ\Ê
UÊÊ V>Ê`iV«iÃ>ÌÊ>vÌiÀÊÎq{Ê`>ÞÃ
UÊÊ>ÕÀiÊÌÊVi>ÀÊL`ÊVÕÌÕÀiÃÊ>vÌiÀÊÇÊ`>ÞÃÊ`iëÌiÊ6>VÞVÊ
ÌÀÕ} ÃÊvÊ£xqÓäÊV}ÉÊ
UÊÊ ÊvÊ6>VÞVÊÃÊÓÊV}É
Unacceptable uses
UÊÊ/Ài>ÌiÌÊvÊVÕÌÞ>VµÕÀi`ÊL>VÌiÀ>Ê«iÕ>Ê­ *®ÊÀÊÃÊ
and soft tissue infections (SSTI) where other more established and
less expensive options are available
UÊÌ>ÊÌ iÀ>«ÞÊvÀÊÀ>«ÃÌÛiÊÀÊÀ>i}>ÌÛiÊviVÌÃ
Dose
UÊÊÈääÊ}Ê6Ê+£ÓÊ >ÃÊLiiÊÃÌÕ`i`ÊvÀÊ *Ê>`Ê--/
UÊÊÈääÊ}Ê6Ê+nÊvÀÊ,-ÊL>VÌiÀi>ÊÃ>Û>}iÊÌ iÀ>«ÞÊÀÊÌ iÀÊ
serious infections
UÊMust adjust for worsening renal function and dialysis (see p. 155 for
dose adjustment recommendation).
Laboratory interactions
UÊÊ ivÌ>ÀiÊ>ÞÊÀiÃÕÌÊÊ«ÃÌÛiÊ`ÀiVÌÊ LýÊÌiÃÌÊÜÌ ÕÌÊ
hemolytic anemia. However, if drug-induced hemolytic anemia is
suspected, discontinue Ceftaroline.
Ceftolozane/tazobactam
Ceftolozane/tazobactam is a novel cephalosporin and β-lactamaseinhibitor combination. It has activity against Gram-negative organisms
and some strains of multi-resistant Pseudomonas spp. It does NOT have
activity against carbapenemase-producing Enterobacteriaceae. It also
has in vitro activity against some streptococci and some Gram-negative
anaerobes, but it does not have reliable Staphylococcus spp. activity.
8
Unacceptable uses
UÊÊ «ÀVÊÌÀi>ÌiÌÊvÊV«V>Ìi`ÊÌÀ>>L`>ÊviVÌÃÊ­V®Ê
or complicated urinary tract infections (cUTI) as current standard
regimens are sufficient for coverage of the typical pathogens involved
in these infections and less expensive options are available
Dose
UÊÊ£°xÊ}Ê6Ê+nÊ >ÃÊLiiÊÃÌÕ`i`ÊvÀÊV1/Ê>`ÊÊVL>ÌÊÜÌ Ê
metronidazole for cIAI
UÊÊ-iÀÕÃÊviVÌÃÊVÕ`}Ê«iÕ>\ÊÎÊ}Ê6Ê+n
UÊÊÕÃÌÊ>`ÕÃÌÊ`ÃiÊvÀÊÜÀÃi}ÊÀi>ÊvÕVÌÊ>`Ê`>ÞÃÃÊ­ÃiiÊ«°£xxÊ
for dose adjustment recommendation).
Colistin (Colistimethate)
Colistin is a polymixin antibiotic. It has in vitro activity against
Acinetobacter spp. and Pseudomonas spp. but does NOT have activity
against Proteus, Serratia, Providentia, Burkholderia, Stenotrophomonas,
Gram-negative cocci, Gram-positive organisms, or anaerobes.
Acceptable uses
UÊÊ>>}iiÌÊvÊviVÌÃÊ`ÕiÊÌÊÕÌ`ÀÕ}ÊÀiÃÃÌ>ÌÊAcinetobacter
and Pseudomonas on a case by case basis.
Unacceptable uses
UÊÊÌ iÀ>«ÞÊvÀÊi«ÀVÊÌÀi>ÌiÌÊvÊÃÕëiVÌi`ÊÀ>i}>ÌÛiÊviVÌÃÊ
Dose
UÊ>`}Ê`Ãi\ÊxÊ}É}ÊVi
UÊÊ>Ìi>ViÊ`Ãi\ÊÓ°xÊ}É}Ê+£ÓÆÊÕÃÌÊ>`ÕÃÌÊvÀÊÜÀÃi}Ê
renal function and dialysis (see p. 155 for dose adjustment
recommendation).
Toxicity
UÊÊ,i>Ê«>ÀiÌ]ÊiÕÀÕÃVÕ>ÀÊLV>`i]ÊiÕÀÌÝVÌÞ
UÊÊÌÀ}\Ê 1 ]ÊVÀi>ÌiÊÌÜViÜiiÞ
9
3.1 Agent-specific guidelines: Antibiotics
Acceptable uses (Cases must be discussed with Infectious Diseases
and Antimicrobial Stewardship Program)
UÊÊ>>}iiÌÊvÊviVÌÃÊ`ÕiÊÌÊÕÌ`ÀÕ}ÊÀiÃÃÌ>ÌÊPseudomonas
spp. infections on a case by case basis
3.1 Agent-specific guidelines: Antibiotics
Hidden Content
- JHH Internal use only
,iviÀiVi\
>`}Ê`ÃiÊvÊVÃÌ\Ê ÊviVÌÊ ÃÊÓä£ÓÆÊx{\£ÇÓäÈ°
Daptomycin
Daptomycin is a lipopeptide antibiotic. It has activity against most strains
of staphylococci and streptococci (including MRSA and VRE). It does
NOT have activity against Gram-negative organisms.
Acceptable uses (Cases must be discussed with Infectious Diseases
and Antimicrobial Stewardship Program)
UÊÊ >VÌiÀi>ÊÀÊi`V>À`ÌÃÊV>ÕÃi`ÊLÞÊ,-ÊÀÊiÌ VÀiÃÃÌ>ÌÊ
coagulase-negative staphylococci in a patient with serious allergy to
Vancomycin
UÊÊ >VÌiÀi>ÊÀÊi`V>À`ÌÃÊV>ÕÃi`ÊLÞÊ,-ÊÊ>Ê«>ÌiÌÊv>}Ê
6>VÞVÊÌ iÀ>«ÞÊ>ÃÊ`iwi`ÊLÞ\Ê
UÊÊ V>Ê`iV«iÃ>ÌÊ>vÌiÀÊÎq{Ê`>ÞÃ
UÊÊ>ÕÀiÊÌÊVi>ÀÊL`ÊVÕÌÕÀiÃÊ>vÌiÀÊÇÊ`>ÞÃÊ`iëÌiÊ6>VÞVÊ
ÌÀÕ} ÃÊvÊ£xqÓäÊV}ÉÊ­ } ÊÀÃÊvÊ >«ÌÞVÊÀiÃÃÌ>ViÆÊ
check Daptomycin MIC and obtain follow up blood cultures)
UÊÊ ÊvÊ6>VÞVÊÃÊÓÊV}É
UÊÊ/ iÀ>«ÞÊvÀÊ6, ÊviVÌÃÊÌ iÀÊÌ >Ê«iÕ>]ÊÊ>ÊV>ÃiÊLÞÊV>ÃiÊL>ÃÃ
Unacceptable uses
UÊÊ >«ÌÞVÊà Õ`Ê "/ÊLiÊÕÃi`ÊvÀÊÌÀi>ÌiÌÊvÊ«iÕ>Ê`ÕiÊÌÊ
its inactivation by pulmonary surfactant.
UÊÊÌ>ÊÌ iÀ>«ÞÊvÀÊÀ>«ÃÌÛiÊviVÌÃÊ
UÊÊ6, ÊVâ>ÌÊvÊÌ iÊÕÀi]ÊÀiëÀ>ÌÀÞÊÌÀ>VÌ]ÊÜÕ`Ã]ÊÀÊ`À>ÃÊ
Dose
UÊÊ >VÌiÀi>\ÊÈq£ÓÊ}É}Ê6Ê+ÊÓ{
UÊÊ `V>À`ÌÃ\ÊÈq£ÓÊ}É}Ê6Ê+ÊÓ{
UÊÊ ÃiÊ>`ÕÃÌiÌÊÃÊiViÃÃ>ÀÞÊvÀÊ À Ê 30 ml/min (see p. 155 for
dose adjustment recommendation).
10
,iviÀiVi\Ê
Daptomycin in S. aureusÊL>VÌiÀi>Ê>`ÊviVÌÛiÊi`V>À`ÌÃ\Ê Ê }ÊÊi`ÊÓääÈÆÊ
Îxx\ÊÈxÎqÈx°
Ertapenem
Ertapenem is a carbapenem antibiotic. It has in vitro activity against
many Gram-negative organisms including those that produce extended
spectrum beta-lactamases (ESBL), but it does not have activity against
Pseudomonas spp. or Acinetobacter spp. Its anaerobic and Grampositive activity is similar to that of other carbapenems, except it does
not have activity against Enteroccocus spp.
Acceptable uses
UÊÊ`ÊÌÊ`iÀ>ÌiÊÌÀ>>L`>ÊviVÌÃÊ­L>ÀÞÊÌÀ>VÌÊviVÌÃ]Ê
diverticulitis, secondary peritonitis/GI perforation)
UÊÊ`iÀ>ÌiÊ`>LiÌVÊvÌÊviVÌÃÊÜÌ ÕÌÊÃÌiÞiÌÃ
UÊÊ`iÀ>ÌiÊÃÕÀ}V>ÃÌiÊviVÌÃÊvÜ}ÊVÌ>>Ìi`Ê«ÀVi`ÕÀi
UÊ*iÛVÊy>>ÌÀÞÊ`Ãi>Ãi
UÊÊ1À>ÀÞÊÌÀ>VÌÊviVÌÃÊV>ÕÃi`ÊLÞÊ - «À`ÕV}ÊÀ}>ÃÃÊ
UÊÊ*Þii« ÀÌÃÊÊ>Ê«>ÌiÌÊÜ ÊÃÊÌÊÃiÛiÀiÞÊ
Unacceptable uses
UÊÊ-iÛiÀiÊviVÌÃÊÊÜ V Pseudomonas spp. are suspected.
Dose
UÊÊ£Ê}Ê6ÊÀÊÊ+Ó{]ÊÕÃÌÊ>`ÕÃÌÊvÀÊÜÀÃi}ÊÀi>ÊvÕVÌÊ>`Ê
dialysis (see p. 155 for dose adjustment recommendation)
Toxicity
UÊÊ >ÀÀ i>]Ê>ÕÃi>]Ê i>`>V i]Ê« iLÌÃÉÌ ÀL« iLÌÃ
Fosfomycin
Fosfomycin is a synthetic, broad-spectrum, bactericidal antibiotic with in
vitro activity against large number of Gram-negative and Gram-positive
organisms including E. coli, Klebsiella spp., Proteus spp., Pseudomonas
spp., and VRE. It does not have activity against Acinetobacter spp.
Fosfomycin is available in an oral formulation only in the U.S. and its
pharmacokinetics allow for one-time dosing.
Acceptable uses
UÊÊ>>}iiÌÊvÊÕV«V>Ìi`Ê1/ÊÊ«>ÌiÌÃÊÜÌ ÊÕÌ«iÊ>ÌLÌVÊ
allergies and/or when no other oral therapy options are available.
11
3.1 Agent-specific guidelines: Antibiotics
Toxicity
UÊÊÞ«>Ì ÞÊ­`iwi`Ê>ÃÊ Ê 10 times the upper limit of normal without
symptoms or 5 times the upper limit of normal with symptoms).
UÊÊ Ã« VÊ«iÕ>
UÊÊÌÀ}\Ê ÊÜiiÞ]ÊÀiÊvÀiµÕiÌÞÊ`ÕÀ}ÊÌ>ÊÌ iÀ>«Þ°Ê
3.1 Agent-specific guidelines: Antibiotics
UÊÊ1V«V>Ìi`Ê1/Ê`ÕiÊÌÊ6,
UÊÊÊ->Û>}iÊÌ iÀ>«ÞÊvÀÊ1/Ê`ÕiÊÌÊÕÌ`ÀÕ}ÊÀiÃÃÌ>ÌÊÀ>i}>ÌÛiÊ
organisms (e.g. Pseudomonas spp.) on case by case basis.
NOTE: Susceptibility to Fosfomycin should be confirmed prior to
initiation of therapy.
Unacceptable uses
UÊÊÃvÞVÊÃ Õ`Ê "/ÊLiÊÕÃi`ÊvÀÊ>>}iiÌÊvÊ>ÞÊviVÌÃÊ
outside of the urinary tract because it does not achieve adequate
concentrations at other sites.
UÊÊ/Ài>ÌiÌÊvÊ>ÃÞ«ÌVÊL>VÌiÀÕÀ>Ê­ÃiiÊ«°Ê££ä®
Dose
UÊÊ1V«V>Ìi`Ê1/\ÊÎÊ}Ê­£ÊÃ>V iÌ®Ê*"ÊVi°Ê
UÊÊ «V>Ìi`Ê1/\ÊÎÊ}Ê­£ÊÃ>V iÌ®Ê*"ÊiÛiÀÞÊ£ÎÊ`>ÞÃÊ­Õ«ÊÌÊÓ£Ê`>ÞÃÊvÊ
treatment)
UÊÊÀiµÕiVÞÊ>`ÕÃÌiÌÊ>ÞÊLiÊiViÃÃ>ÀÞÊÊ«>ÌiÌÃÊÜÌ Ê À Ê 50
mL/min. Contact the ID Pharmacist for dosing recommendations.
UÊÊ*Ü`iÀÊà Õ`ÊLiÊÝi`ÊÜÌ Êäq£ÓäÊÊvÊVÊÜ>ÌiÀ]ÊÃÌÀÀi`ÊÌÊ
dissolve and administered immediately.
Toxicity
UÊÊ >ÀÀ i>]Ê>ÕÃi>]Ê i>`>V i]Ê`ââiÃÃ]Ê>ÃÌ i>Ê>`Ê`Þëi«Ã>
Linezolid
Acceptable uses
UÊÊ VÕiÌi`Ê6>VÞVÊÌiÀi`>ÌiÊStaphylococcus aureus (VISA)
or Vancomycin resistant Staphylococcus aureus (VRSA) infection
UÊÊ VÕiÌi`Ê,-ÊÀÊiÌ VÀiÃÃÌ>ÌÊV>}Õ>Ãii}>ÌÛiÊ
staphylococcal infection in a patient with serious allergy to Vancomycin
UÊÊ VÕiÌi`Ê,-ÊÀÊiÌ VÀiÃÃÌ>ÌÊV>}Õ>Ãii}>ÌÛiÊ
staphylococcal infection in a patient failing Vancomycin therapy (as
`iwi`ÊLiÜ®\Ê
UÊÊ >VÌiÀi>Éi`V>À`ÌÃ\Êv>ÕÀiÊÌÊVi>ÀÊL`ÊVÕÌÕÀiÃÊ>vÌiÀÊ
ÇÊ`>ÞÃÊ`iëÌiÊ6>VÞVÊÌÀÕ} ÃÊvÊ£xqÓäÊV}É°Ê- Õ`ÊLiÊ
used in combination with another agent
UÊÊ*iÕ>\ÊÜÀÃi}ÊwÌÀ>ÌiÊÀÊ«Õ>ÀÞÊÃÌ>ÌÕÃÊÊ>Ê«>ÌiÌÊ
with documented MRSA pneumonia after 2 to 3 days or if the
MIC of Vancomycin is 2 mcg/mL, or if achieving appropriate
vancomycin trough is unlikely (e.g., obesity)
UÊÊ >ÃiÃÊÃ Õ`ÊLiÊ`ÃVÕÃÃi`ÊÜÌ ÊviVÌÕÃÊ Ãi>ÃiÃÊÀÊ
Antimicrobial stewardship
UÊHigh suspicion of CA-MRSA necrotizing pneumonia in a seriously
ill patient
12
Dose
UÊÊÈääÊ}Ê6É*"Ê+£Ó
UÊÊ-Ê>`ÊÃÃÌÀÕVÌÕÀiÊviVÌÃ\Ê{ääÊ}Ê6É*"Ê+£Ó
Toxicity
UÊÊ iÊ>ÀÀÜÊÃÕ««ÀiÃÃÊ­ÕÃÕ>ÞÊVVÕÀÃÊÜÌ ÊwÀÃÌÊÓÊÜiiÃÊvÊÌ iÀ>«Þ®
UÊÊ"«ÌVÊiÕÀÌÃÊ>`ÊÀÀiÛiÀÃLiÊÃiÃÀÞÊÌÀÊ«ÞiÕÀ«>Ì ÞÊ­ÕÃÕ>ÞÊ
occurs with prolonged therapy > 28 days)
UÊÊ >ÃiÊÀi«ÀÌÃÊvÊ>VÌVÊ>V`ÃÃ
UÊÊ >ÃiÊÀi«ÀÌÃÊvÊÃiÀÌÊÃÞ`ÀiÊÜ iÊV>`ÃÌiÀi`ÊÜÌ Ê
serotonergic agents (SSRIs, TCAs, MAOIs, etc.)
UÊÊÌÀ}\Ê
ÊÜiiÞ
Tigecycline
Tigecycline is a tetracycline derivative called a glycylcycline. It has in
vitro activity against most strains of staphylococci and streptococci
(including MRSA and VRE), anaerobes, and many Gram-negative
organisms with the exception of Proteus spp. and Pseudomonas
aeruginosa. It is FDA approved for skin and skin-structure infections and
intra-abdominal infections.
NOTE: Peak serum concentrations of Tigecycline do not exceed
1 mcg/mL which limits its use for treatment of bacteremia
Acceptable uses
UÊÊ>>}iiÌÊvÊÌÀ>>L`>ÊviVÌÃÊÊ«>ÌiÌÃÊÜÌ Ê
contraindications to both beta-lactams and fluoroquinolones
UÊÊ>>}iiÌÊvÊviVÌÃÊ`ÕiÊÌÊÕÌ`ÀÕ}ÊÀiÃÃÌ>ÌÊÀ>i}>ÌÛiÊ
organisms including Acinetobacter spp. and Stenotrophomonas
maltophilia on a case by case basis
UÊÊ->Û>}iÊÌ iÀ>«ÞÊvÀÊ,-É6, ÊviVÌÃÊÊ>ÊV>ÃiÊLÞÊV>ÃiÊL>ÃÃ
Dose
UÊÊ£ääÊ}Ê6ÊVi]ÊÌ iÊxäÊ}Ê6Ê+£Ó
UÊÊ£ääÊ}Ê6ÊVi]ÊÌ iÊÓxÊ}Ê6Ê+£ÓÊvÊÃiÛiÀiÊ i«>ÌVÊ«>ÀiÌÊ
­ `ÊÊ*Õ} Ê£äq£x®
Toxicity
UÊÊ >ÕÃi>Ê>`ÊÛÌ}Ê
13
3.1 Agent-specific guidelines: Antibiotics
UÊ ÊÊ VÕiÌi`Ê6, ÊviVÌÊ
UÊÊÀ>«ÃÌÛiÊVVVÊÊV >ÃÊÊL`ÊVÕÌÕÀiÃÊÊ>Ê 1]ÊÀÊV}ÞÊ
transplant patient known to be colonized with VRE
Unacceptable uses
UÊÊ*À« Þ>ÝÃ
UÊÊÌ>ÊÌ iÀ>«ÞÊvÀÊÃÌ>« ÞVVV>ÊviVÌ
UÊÊ6, ÊVâ>ÌÊvÊÌ iÊÃÌ]ÊÕÀi]ÊÀiëÀ>ÌÀÞÊÌÀ>VÌ]ÊÜÕ`Ã]ÊÀÊ`À>Ã
3.1 Agent-specific guidelines: Antibiotics
Trimethoprim/sulfamethoxazole
(Bactrim®, TMP/SMX)
Trimethoprim/sulfamethoxazole is a sulfonamide antibiotic. It has in vitro
activity against Enterobacteriaceae spp., B. cepacia, S. maltophilia,
Acinetobacter spp., Achromobacter spp., Nocardia spp., Listeria,
Pneumocystis jirovecii (PCP), staphylococci (including S. aureus and
Coagulase-negative staph), but does NOT cover Pseudomonas spp.
It has variable activity against streptococci and no activity against
anaerobes.
Acceptable uses
UÊ1À>ÀÞÊÌÀ>VÌÊviVÌÃÊ­1/®
UÊS. aureus skin and soft-tissue infections (SSTI)
UÊPneumocystis jirovecii pneumonia (PCP) treatment and prophylaxis
UÊS. maltophilia infections
UÊ V>À`>ÊviVÌÃÊ
UÊÀ>i}>ÌÛiÊL>VÌiÀi>ÊÜ iÊÀ}>ÃÊÃÊÃÕÃVi«ÌLiÊ
UÊÊ->Û>}iÊÌ iÀ>«ÞÊvÀÊ,-ÊL>VÌiÀi>ÊÊVL>ÌÊÜÌ Ê>Ì iÀÊ
agent
UÊÊ «ÀVÊVÛiÀ>}iÊvÊListeria meningitis in patients with penicillin
allergies
UÊÊ-Õ««ÀiÃÃÛiÊÌ iÀ>«ÞÊ>`ÊÊÃiÊV>ÃiÃÊÌÀi>ÌiÌÊvÀÊLiÊ>`ÊÌÊ
infections
Unacceptable uses
UÊÌ iÀ>«ÞÊvÀÊS. aureus bacteremia
Dose
UÊTrimethoprim/sulfamethoxazole dosing is based on trimethoprim
component
UÊ/*É-8Ê >ÃÊiÝViiÌÊL>Û>>LÌÞ]ÊÌ ÕÃÊVÛiÀÃÊvÀÊ6ÊÌÊ*"Ê
ÃÊ£\£Ê­näÉ{ääÊ}Ê6ÊrÊ£Ê--ÊÌ>LÆÊ£ÈäÉnääÊ}Ê6ÊrÊ£Ê -ÊÌ>L®Ê
UÊ1ÃiÊ>`ÕÃÌi`Ê 7rÊQ 7ʳÊä°{Ê­ 7ÊÊ 7®RÊÊLiÃiÊ«>ÌiÌÃÊ­Îä¯Ê
over IBW)
Treatment
UÊ1/\Ê£Ê -ÊÌ>LÊ+£ÓÊ
UÊ--/\Ê£ÓÊ -ÊÌ>LÊ+£Ó
UÊ* *\£xÓäÊ}É}É`>ÞÊ­Ê`Û`i`Ê`ÃiÃ]Ê+È+n®
UÊ,-ÊL>VÌiÀi>\£ä£xÊ}É}É`>ÞÊ­Ê`Û`i`Ê`ÃiÃ]Ê+È+n®
UÊS. maltophiliaÊviVÌÃ\£xÊ}É}É`>ÞÊ­Ê`Û`i`Ê`ÃiÃ]Ê+È+n®Ê
14
Prophylaxis
UÊ* *\Ê£Ê--Ê`>ÞÊÀÊ£Ê -ÊÎÊÌiÃÉÜiiÊ
UÊ/Ý«>ÃÃÃ\Ê£Ê -Ê`>ÞÊ
Toxicity
UÊ \Ê Þ«iÀÃiÃÌÛÌÞÊ­£°Èn¯®]ÊÕ«ÃiÌ]Ê«ÃiÕ`ÊiiÛ>ÌÊÊ
VÀi>ÌiÊ­£n¯®Ê
UÊ ÊÜÌ Ê } iÀÊ`ÃiÃ\Ê Þ«iÀ>i>]ÊÞiÃÕ««ÀiÃÃ
UÊ"VV>Ã>\Êi« ÀÌÝVÌÞ]Ê« ÌÃiÃÌÛÌÞ]ÊiÌ i}Li>Ê­ÜÌ Ê
severe G6PD deficiency)
UÊ,>Ài\Ê>Ãi«ÌVÊi}ÌÃ]Ê i«>ÌÌÝVÌÞ]ÊÌÝVÊi«`iÀ>ÊiVÀÞÃÃÊ
(TEN), SJS, Sweet’s syndrome
Drug Interaction
UÊ7>Àv>À]ÊiÌ ÌÀiÝ>Ìi]Ê« iÞÌ]Ê`}Ý]ÊÃÕvÞÕÀi>Ã]Ê
procainamide, oral contraceptives
15
3.1 Agent-specific guidelines: Antibiotics
UÊ V>À`>ÊviVÌÃ\Ê£xÊ}É}É`>ÞÊ­Ê`Û`i`Ê`ÃiÃ]Ê+È+n®ÆÊÜiÀÊ
doses (5-10 mg/kg/day) can be used after several weeks of therapy
or cutaneous infections
UÊi}ÌÃ\ÊÓäÊ}É}É`>ÞÊ­Ê`Û`i`Ê`ÃiÃ]Ê+È®
UÊ"Ì iÀÊviVÌÃ\Ên£äÊ}É}É`>ÞÊ­Ê`Û`i`Ê`ÃiÃ]Ê+È£Ó®
UÊÕÃÌÊ>`ÕÃÌÊ`ÃiÊvÀÊÜÀÃi}ÊÀi>ÊvÕVÌÊ>`Ê`>ÞÃÃÊ­ÃiiÊ«°£xxÊ
for dose adjustment recommendation).
3.2 Agent-specific guidelines: Antifungals
Antifungals
Liposomal Amphotericin B (AmBisome®)
NOTES:
UÊÊ Ã}ÊvÊ ÃiÊ>`Ê« ÌiÀVÊ Ê`iÝÞV >ÌiÊÃÊ
significantly different. Do not use AmBisome doses when
ordering Amphotericin B deoxycholate and vice versa.
UÊÊ« ÌiÀVÊ Ê`iÝÞV >ÌiÊÃÊ«ÀiviÀÀi`ÊÊ«>ÌiÌÃÊÜÌ Êi`
stage renal disease on dialysis who are anuric.
AmBisome, like all Amphotericin B products, has broad spectrum
antifungal activity with in vitro activity against Candida, Aspergillus,
Zygomycosis and Fusarium.
Acceptable uses
UÊ >``>Êi`«Ì >ÌÃ]Êi`V>À`ÌÃ]Ê -ÊviVÌqwÀÃÌÊiÊÌ iÀ>«Þ
UÊ ÀÞ«ÌVVVÕÃÊi}ÌÃwÀÃÌÊiÊÌ iÀ>«ÞÊÊ
UÊ<Þ}ÞVÃiÃÊ­Mucor, Rhizopus, Cunninghamella®qwÀÃÌÊiÊÌ iÀ>«ÞÊ
UÊÊ iÕÌÀ«iVÊviÛiÀÊvÊÀiViÛ}Ê6ÀV>âiÊÀÊ*Ã>V>âiÊ
prophylaxis
UÊÌiÀ>ÌÛiÊÌÀi>ÌiÌÊvÊÛ>ÃÛiÊ>ëiÀ}ÃÃ
UÊÊÌiÀ>ÌÛiÊÌÀi>ÌiÌÊvÊV>``i>]ÊV>``>Ê«iÀÌÌÃÊ
Dose
UÊÊ >``i>]Ê ÃÌ«>ÃÃÃ]ÊÌ iÀÊÛ>ÃÛiÊV>``>ÊviVÌÃ\Ê
3 mg/kg/day
UÊÊ >``>Êi`«Ì >ÌÃ]Êi`V>À`ÌÃ]Ê -ÊviVÌ]ÊC. krusei
V>``i>\ÊxÊ}É}É`>Þ
UÊÛ>ÃÛiÊw>iÌÕÃÊvÕ}\ÊxÊ}É}É`>Þ
UÊ iÕÌÀ«iVÊviÛiÀ]ÊV>``i>ÊÊiÕÌÀ«iVÊ«>ÌiÌ\ÊÎqxÊ}É}É`>Þ
UÊ ÀÞ«ÌVVV>Êi}ÌÃ\ÊÎq{Ê}É}É`>Þ
Toxicity
UÊvÕÃÀi>Ìi`ÊÀi>VÌÃ\ÊviÛiÀ]ÊV Ã]ÊÀ}ÀÃ]Ê Þ«ÌiÃ
UÊÊ,i>Ê«>ÀiÌÊ­i >Vi`ÊÊ«>ÌiÌÃÊÜÌ ÊVVÌ>ÌÊi« ÀÌÝVÊ
drugs)
UÊ iVÌÀÞÌiÊL>>ViÃ
UÊÊ*Õ>ÀÞÊÌÝVÌÞÊ­V iÃÌÊ«>]Ê Þ«Ý>]Ê`Þëi>®]Ê>i>]ÊiiÛ>ÌÊÊ
hepatic enzymes-rare
UÊÊÌÀ}\Ê 1 ÉVÀi>Ìi]Ê]Ê}]Ê* ÃÊ>ÌÊL>ÃiiÊ>`Ê`>ÞÊÊ
ëÌ>âi`Ê«>ÌiÌÃÆÊ-/É/Ê>ÌÊL>ÃiiÊ>`ÊiÛiÀÞÊ£ÓÊÜiiÃÊ
16
Aspergillosis
UÊVVi«Ì>LiÊÕÃiÃ
UÊÊÊVL>ÌÊÜÌ Ê6ÀV>âiÊvÀÊVwÀi`ÊÛ>ÃÛiÊ
aspergillosis (see p. 133)
UÊÊ,ivÀ>VÌÀÞÊ`Ãi>ÃiÊvÀÊÕÃiÊÊVL>ÌÊÜÌ Ê6ÀV>âi]Ê
Posaconazole or AmBisome® for confirmed invasive aspergillosis.
UÊ1>VVi«Ì>LiÊÕÃiÃ
UÊÊV>vÕ}Ê>iÊÀÊÊVL>ÌÊÜÌ ÊÌ iÀÊ>ÌvÕ}>Ê>}iÌÃÊÃÊ
not recommended for empiric therapy in patients with CT findings
suggestive of aspergillosis (e.g., possible aspergillosis) without
plans for diagnostic studies.
UÊÊV>vÕ}Ê`iÃÊÌÊ >ÛiÊ}`Êin vitro activity against
zygomycoses (Mucor, Rhizopus, Cunninghamella, etc.).
Candidiasis
UÊVVi«Ì>LiÊÕÃiÃ
UÊ/Ài>ÌiÌÊvÊÛ>ÃÛiÊV>``>ÃÃÊ`ÕiÊÌÊC. glabrata or C. krusei.
UÊÊ/Ài>ÌiÌÊvÊÛ>ÃÛiÊV>``>ÃÃÊÊ«>ÌiÌÃÊÜ Ê>ÀiÊ "/ÊVV>ÞÊ
stable due to candidemia or have received prior long-term azole
therapy.
UÊÌiÀ>ÌÛiÊÌÀi>ÌiÌÊvÊÀiVÕÀÀiÌÊië >}i>ÊV>``>Ãð
UÊÌiÀ>ÌÛiÊÌÀi>ÌiÌÊvÊi`V>À`Ìð
UÊ1>VVi«Ì>LiÊÕÃiÃ
UÊÊV>vÕ}Ê >ÃÊ«ÀÊ«iiÌÀ>ÌÊÌÊÌ iÊ -Ê>`ÊÕÀ>ÀÞÊÌÀ>VÌ°ÊÌÊ
should be avoided for infections involving those sites.
Neutropenic fever
UÊÊV>vÕ}ÊV>ÊLiÊÕÃi`ÊvÀÊiÕÌÀ«iVÊviÛiÀÊÊ«>ÌiÌÃÊÜ Ê>ÀiÊÌÊ
suspected to have aspergillosis or zygomycosis.
Dose
UÊÊ >``i>]ÊÛ>ÃÛiÊV>``>ÃÃ]ÊiÕÌÀ«iVÊviÛiÀ\Ê£ääÊ}Ê6Ê
Q24H
UÊ >``>Êi`V>À`ÌÃ\Ê£xäÊ}Ê6Ê+Ó{
UÊ,iVÕÀÀiÌÊië >}i>ÊV>``>ÃÃ\Ê£xäÊ}Ê6Ê+Ó{
UÊÛ>ÃÛiÊ>ëiÀ}ÃÃ\Ê£ääq£xäÊ}Ê6Ê+Ó{
UÊ"LiÃiÊ«>ÌiÌÃ
UÊÊ£ääq£xäÊ}\Ê£xäÊ}Ê6Ê+Ó{
UÊÊ> £xäÊ}\Ê ÃÕÌÊ Ê* >À>VÃÌ
Drug Interactions
UÊÊ ÃiÊÌÀ}ÊÃÊÀiVi`i`ÊÜ iÊV>vÕ}ÊÃÊÕÃi`ÊÜÌ ÊÌ iÊ
vÜ}Ê>}iÌÃÊVVÌ>ÌÞ\
17
3.2 Agent-specific guidelines: Antifungals
Micafungin
NOTE: Micafungin does not have activity against Cryptococcus.
3.2 Agent-specific guidelines: Antifungals
UÊÊ-ÀÕÃÊqÊiÛiÃÊvÊ-ÀÕÃÊ>ÞÊLiÊVÀi>Ãi`]ÊÌÀÊvÀÊ
Sirolimus toxicity
UÊÊ vi`«iÊqÊiÛiÃÊvÊ vi`«iÊ>ÞÊLiÊVÀi>Ãi`]ÊÌÀÊvÀÊ
Nifedipine toxicity
UÊÊÌÀ>V>âiÊqÊiÛiÃÊvÊÌÀ>V>âiÊ>ÞÊLiÊVÀi>Ãi`]ÊÌÀÊvÀÊ
Itraconazole toxicity
Toxicity
UÊÊvÕÃÀi>Ìi`ÊÀi>VÌÃÊ­À>à ]Ê«ÀÕÀÌî]Ê« iLÌÃ]Ê i>`>V i]Ê>ÕÃi>Ê
and vomiting, and elevations in hepatic enzymes.
UÊÌÀ}\Ê-/É/ÉLÀÕLÊ>ÌÊL>ÃiiÊ>`ÊiÛiÀÞÊ£qÓÊÜiiÃÊ>vÌiÀ°
Posaconazole
Posaconazole is a broad spectrum azole anti-fungal agent. It has in vitro
activity against Candida, Aspergillus, Zygomycosis and Fusarium spp.
Acceptable uses
UÊ/Ài>ÌiÌÊvÊÛ>ÃÛiÊâÞ}ÞVÃÃÊÊVL>ÌÊÜÌ Ê« ÌiÀVÊ
UÊÊÌ iÀ>«ÞÊvÀÊâÞ}ÞVÃÃÊ>vÌiÀÊÇÊ`>ÞÃÊvÊVL>ÌÊÌ iÀ>«ÞÊ
with Amphotericin B
UÊ*À« Þ>ÝÃÊÊ«>ÌiÌÃÊÜÌ Ê i>Ì}VÊ>}>VÞ
UÊ/Ài>ÌiÌÊvÊ>ëiÀ}ÃÃÊÊ«>ÌiÌÃÊÜÌ Ê6ÀV>âiÊÌiÀ>Vi
Unacceptable uses
UÊ >``>ÃÃÉ iÕÌÀ«iVÊviÛiÀ
UÊÀÃÌiÊÌÀi>ÌiÌÊvÊ>ëiÀ}ÃÃ
Dose
"/ -\Ê
UÊÊ >V Ê`ÃiÊvÊÃÕëiÃÊà Õ`ÊLiÊ}ÛiÊÜÌ Ê>ÊvÕÊi>ÊÀÊÜÌ ÊµÕ`Ê
nutritional supplements if patients cannot tolerate full meals. Can also
be given with an acidic beverage (e.g. ginger ale).
UÊÊ i>Þi`ÊÀii>ÃiÊÌ>LiÌÃÊ>`ÊÀ>ÊÃÕëiÃÊV>ÌÊLiÊÕÃi`Ê
interchangeably due to differences in the dosing of each formulation.
Prophylaxis
UÊ"À>Ê-ÕëiÃ\ÊÓääÊ}Ê*"Ê+n
UÊ ÝÌi`i`Ê,ii>ÃiÊ/>LiÌ\ÊÎääÊ}Ê*"Ê`>Þ
Treatment
UÊÊ"À>Ê-ÕëiÃ\ÊÓääÊ}Ê*"Ê+ÈÊvÀÊÇÊ`>ÞÃ]ÊÌ iÊ{ääÊ}Ê*"Ê
Q8-Q12H
UÊÊ ÝÌi`i`Ê,ii>ÃiÊ/>LiÌ\ÊÎääÊ}Ê*"Ê+£ÓÊvÀÊ£Ê`>Þ]ÊÌ iÊÎääÊ}Ê
PO daily
18
Drug Interactions: See Table on p. 21
Toxicity
UÊÊÊÕ«ÃiÌÊ­H{䯮]Ê i>`>V iÃ]ÊiiÛ>ÌÊÊ i«>ÌVÊiâÞiðÊ,>ÀiÊLÕÌÊ
serious effects include QTc prolongation.
UÊÊÌÀ}\Ê-/É/ÉLÀÕLÊ>ÌÊL>ÃiiÊ>`ÊiÛiÀÞÊ£qÓÊÜiiÃÊ>vÌiÀ
,iviÀiViÃ\
V>ÊivwV>VÞÊvÊiÜÊ>ÌvÕ}>Ê>}iÌÃ\Ê ÕÀÀÊ"«ÊVÀL°ÊÓääÈÆ\{nÎnn°
*Ã>V>âi\Ê>ÊLÀ>`ÊëiVÌÀÕÊÌÀ>âiÊ>ÌvÕ}>\Ê>ViÌÊviVÌÊ Ã°ÊÓääxÆÊx\ÇÇxnx°
Voriconazole
NOTE: Voriconazole does not cover zygomycoses (Mucor,
Rhizopus, Cunninghamella, etc.).
Acceptable uses
UÊAspergillosis
UÊScedosporium apiospermum
UÊProphylaxis in patients with hematologic malignancy
Unacceptable uses
UÊÊCandidiasis / Neutropenic fever
Voriconazole should not be used as first-line therapy for the treatment
of candidiasis or for empiric therapy in patients with neutropenic fever.
Dose
UÊÊ>`}Ê`Ãi\ÊÈÊ}É}Ê6É*"Ê+£ÓÊÝÊÓÊ`ÃiÃ
UÊ>Ìi>ViÊ`Ãi\Ê{Ê}É}Ê6É*"Ê+£Ó
UÊÊ ÃiÊ>`ÕÃÌiÌÊÃÊiViÃÃ>ÀÞÊvÀÊ i«>ÌVÊÃÕvwViVÞ\
UÊ `ÊÊ*Õ} Ê­ÊÀÊ ®\Ê↓ >Ìi>ViÊ`ÃiÊLÞÊxä¯
UÊÊ `ÊÊ*Õ} Ê­ ®\Ê1ÃiÊÞÊvÊLiiwÌÃÊÕÌÜi} ÊÀÃÃ°Ê ÃÕÌÊ
ID pharmacist for dose adjustment recommendations.
UÊÊ ÃiÊiÃV>>ÌÊ>ÞÊLiÊiViÃÃ>ÀÞÊvÀÊÃiÊ«>ÌiÌÃÊ`ÕiÊÌÊ
subtherapeutic levels.
UÊÊ ÃiÊL>Ãi`ÊÊ>VÌÕ>ÊL`ÞÊÜi} ÌÊÕiÃÃÊ«>ÌiÌÊÎä¯ÊÛiÀÊ 7ÆÊ
then use adjusted body weight. (Adj. BW).
`°Ê 7ÊrÊQ 7ʳÊä°{Ê­ 7ÊÊ 7®R
IBW - Ideal Body Weight
ABW - Actual Body Weight
19
3.2 Agent-specific guidelines: Antifungals
Therapeutic monitoring:
UÊ*Ã>V>âiÊÌÀÕ} ÊiÛiÃÊÃ Õ`ÊLiÊVÃ`iÀi`ÊÊ«>ÌiÌÃÊÜ Ê>Ài\
UÊ ÌÊÀië`}ÊÌÊÌ iÀ>«ÞÊvÀÊ>ÌÊi>ÃÌÊÇÊ`>ÞÃ
UÊ i}ÊÌÀi>Ìi`ÊvÀÊÕVÊÀÊiÃÃÊÃÕÃVi«ÌLiÊÀ}>ÃÃ
UÊ Ý«iÀiV}ÊÕVÃÌÃÊÀÊ>>LÃÀ«ÌÊÃÞ`Ài
UÊ1>LiÊÌÊVÃÕiÊ } Êv>ÌÊi>ÃÊ­vÊÀiViÛ}ÊÌ iÊÃÕëiî
3.2 Agent-specific guidelines: Antifungals
Therapeutic monitoring
UÊÊ6ÀV>âiÊÌÀÕ} ÊiÛiÃÊÃ Õ`ÊLiÊVÃ`iÀi`ÊÊ«>ÌiÌÃÊÜ Ê>Ài\
UÊÊ ÌÊÀië`}ÊÌÊÌ iÀ>«ÞÊ>vÌiÀÊ>ÌÊi>ÃÌÊxÊ`>ÞÃÊvÊÌ iÀ>«ÞÊÕÃ}Ê>Ê
mg/kg dosing strategy
UÊÊ,iViÛ}ÊVVÌ>ÌÊ`ÀÕ}ÃÊÌ >ÌÊ>ÞÊVÀi>ÃiÊÀÊ`iVÀi>ÃiÊ
Voriconazole levels
UÊÊ Ý«iÀiV}Ê>`ÛiÀÃiÊiÛiÌÃÊ`ÕiÊÌÊ6ÀV>âi
UÊÊ Ý«iÀiV}ÊÊ`ÞÃvÕVÌ
UÊÊ6ÀV>âiÊÌÀÕ} ÊiÛiÃÊÃ Õ`ÊLiÊLÌ>i`ÊxqÇÊ`>ÞÃÊ>vÌiÀÊÃÌ>ÀÌÊvÊ
Ì iÀ>«ÞÊ­«iÀvÀi`Êq®°
UÊÊ>ÊÌÀÕ} \ÊÓqx°xÊV}É°ÊiÛiÃÊÊ£ÊV}ÉÊ >ÛiÊLiiÊ
associated with clinical failures and levels >5.5 mcg/mL with toxicity.
Drug Interactions: See Table on p. 21
Toxicity
UÊÊ6ÃÕ>Ê`ÃÌÕÀL>ViÃÊ­HÎ䯮ÊÕÃÕ>ÞÊÃivÌi`]ÊÀ>à ]ÊviÛiÀ]ÊiiÛ>ÌÃÊ
in hepatic enzymes.
UÊÊÌÀ}\Ê-/É/ÉLÀÕLÊ>ÌÊL>ÃiiÊ>`ÊiÛiÀÞÊ£qÓÊÜiiÃÊ>vÌiÀ
,iviÀiViÃ\
6ÀVâi\Ê ÊviVÌÊ ÃÊÓääÎÆÊÎÈ\ÈÎä°
6ÀV>âiÊÊiÕÌÀ«iVÊviÛiÀ\Ê Ê }ÊÊi`ÊÓääÓÆÎ{È­{®\ÓÓx°Ê
6ÀV>âiÊ/ \Ê ÊviVÌÊ ÃÊÓäänÆÊ{È\Ó䣰
Azole drug interactions
The following list contains major drug interactions involving drug
metabolism and absorption. This list is not comprehensive and is
intended as a guide only. You must check for other drug interactions
when initiating azole therapy or starting new medication in patients
already receiving azole therapy.
Drug metabolism:
ÞÌV ÀiÊ­ 9*®Ê*{xäÊ LÌÀÃ\Ê`iVÀi>ÃiÊÌ iÊiÌ>LÃÊvÊViÀÌ>Ê
drugs (CYP450 substrates) resulting in increased drug concentrations in
the body (occurs immediately)
ÞÌV ÀiÊ­ 9*®Ê*{xäÊ`ÕViÀÃ\ÊVÀi>ÃiÊÌ iÊiÌ>LÃÊvÊViÀÌ>Ê
drugs (CYP450 substrates) resulting in decreased drug concentrations
in the body (may take up to 2 weeks for upregulation of enzymes to
occur)
Drug absorption/penetration:
*}ÞV«ÀÌiÊ­*}«®Ê LÌÀ\Ê`iVÀi>ÃiÊÌ iÊvÕVÌÊvÊÌ iÊivyÕÝÊ«Õ«]Ê
resulting in increased absorption/penetration of P-gp substrates
*}ÞV«ÀÌiÊ`ÕViÀ\ÊVÀi>ÃiÊÌ iÊvÕVÌÊvÊÌ iÊivyÕÝÊ«Õ«]Ê
resulting in decreased absorption/penetration of P-gp substrates
PotencyÊvÊ ÞÌV ÀiÊ*{xäÊ LÌ\Ê6ÀV>âiÊÊÌÀ>V>âiÊÊ
Posaconazole > Fluconazole
20
Do not use
Recommendations
↓ cyclosporine dose to 3⁄4 and monitor levels
May ↓ posaconazole concentrations when using suspension
Consider dose reducing
↓ tacrolimus dose to 1⁄3 and monitor levels
Avoid concomitant use unless benefit outweighs risk
If used together, monitor effects of drugs and consider decreasing dose
when posaconazole is added
Amiodarone, atazanavir, digoxin, erythromycin, all calcium channel blockers, Monitor effect of drugs and consider decreasing dose when
ritonavir, statins (avoid lovastatin and simvastatin), vinca alkaloids
posaconazole is added
Drug
ÞÊ«ÀiÃVÀLi`\ sirolimus
iÃÃÊVÞÊ«ÀiÃVÀLi`\ cisapride, ergot alkaloids, pimozide,
quinidine, triazolam
Cyclosporine
Metoclopramide, proton pump inhibitors
Midazolam
Tacrolimus
Cimetidine, efavirenz, phenytoin, rifabutin, rifampin
Warning/precaution
Drug
ÞÊ«ÀiÃVÀLi`\ statins (lovastatin, simvastatin)
iÃÃÊVÞÊ«ÀiÃVÀLi`\ cisapride, dofetilide, ergot alkaloids,
nisoldipine, oral midazolam, pimozide, quinidine, triazolam
ÞÊ«ÀiÃVÀLi`\ atorvastatin, benzodiazepines, chemotherapy
(busulfan, docetaxel, vinca alkaloids), cyclosporine, digoxin, efavirenz,
eletriptan, fentanyl, oral hypoglycemics, indinavir, IV midazolam,
nifedipine, ritonavir, saquinavir, sirolimus, tacrolimus, verapamil, steroids
(budesonide, dexamethasone, fluticasone, methylprednisolone), warfarin
iÃÃÊVÞÊ«ÀiÃVÀLi`\ alfentanil, buspirone, cilostazol, disopyramide,
felodipine, trimetrexate
ÞÊ«ÀiÃVÀLi`\ carbamazepine, efavirenz, isoniazid, nevirapine,
phenobarbital, phenytoin, rifabutin, rifampin, antacids, H2 receptor
antagonists, proton pump inhibitors
Clarithromycin, erythromycin, fosamprenavir, indinavir, ritonavir, saquinavir
Do not use
Recommendations
↓ plasma concentration of itraconazole, if possible avoid concomitant
use or monitor itraconazole levels
plasma concentration of the interacting drug, monitor levels when
possible, monitor for drug toxicity and consider dose reduction
3.2 Agent-specific guidelines: Antifungals
plasma concentration of itraconazole, monitor itraconazole levels and
monitor for toxicity
↓
Contraindicated
ITRACONAZOLE and major metabolite hydroxyitraconazole (substrate and inhibitor of CYP3A4 and P-gp efflux)
Warning/precaution
Contraindicated
POSACONAZOLE (substrate and inhibitor for P-gp efflux, inhibitor of CYP3A4)
↓
21
Do not use
Recommendations
3.2 Agent-specific guidelines: Antifungals
↓ cyclosporine dose to 1⁄2 and monitor levels
voriconazole dose to 5 mg/kg IV/PO Q12H and ↓ efavirenz to 300 mg
PO daily
Tacrolimus
↓ tacrolimus dose to 1⁄3 and monitor levels
Sirolimus
↓ÊÃÀÕÃÊ`ÃiÊLÞÊÇx¯Ê>`ÊÌÀÊiÛiÃ
Omeprazole
↓ omeprazole dose to 1⁄2
Maraviroc
↓ maraviroc dose to 150 mg twice daily
Methadone
Monitor effect of the interacting drug and consider decreasing dose
Phenytoin
voriconazole to 5 mg/kg IV/PO Q12H and monitor levels
Ritonavir low dose (100 mg Q12H)
Avoid this combination unless benefits outweigh risks
Warfarin
Monitor INR levels
ÞÊ«ÀiÃVÀLi`\ all benzodiazepines (avoid midazolam and triazolam), Monitor effect of drugs and consider decreasing dose when voriconazole
all calcium channel blockers, fentanyl, oxycodone & other long acting opioids, is added
NSAIDs, oral contraceptives, statins (avoid lovastatin and simvastatin),
sulfonylureas, vinca alkaloids, pomalidomide, simeprevir, boceprevir, telaprevir
iÃÃÊVÞÊ«ÀiÃVÀLi`\ alfentanil
Drug
ÞÊ«ÀiÃVÀLi`\ carbamazepine, rifabutin, rifampin,
ritonavir 400 mg Q12H
iÃÃÊVÞÊ«ÀiÃVÀLi`\ long-acting barbiturates, cisapride,
ergot alkaloids, pimozide, quinidine, St. John’s Wort
Cyclosporine
Efavirenz
Contraindicated
Warning/precaution
Drug
Cisapride
ÞÊ«ÀiÃVÀLi`\ cyclosporine, glipizide, glyburide, phenytoin,
rifabutin, tacrolimus, warfarin
iÃÃÊVÞÊ«ÀiÃVÀLi`\ oral midazolam, theophylline, tolbutamide
Rifampin
Recommendations
↓ plasma concentration of fluconazole, consider increasing fluconazole dose
Do not use
plasma concentration of the interacting drug, monitor levels when
possible, monitor for drug toxicity and consider dose reduction
↓
FLUCONAZOLE (substrate of CYP3A4 and inhibitor of CYP3A4, CYP2C9, and CYP2C19, interactions are often dose dependent)
Warning/precaution
Contraindicated
VORICONAZOLE (substrate and inhibitor of CYP2C19, CYP2C9, and CYP3A4)
↓
↓
22
Indications for pneumococcal vaccines for adults ≥ 19 years of age
Risk group
All adults ≥ 65 years of age
CSF leak or cochlear implants
Functional or anatomic asplenia
Prevnar 13®
Yes
Yes
Yes
Immunocompetent persons with certain No
chronic medical conditions (e.g. heart
disease*, lung disease†, liver disease,
DM), alcoholism, cigarette smoking
ÕV«ÀÃi`Ê ÃÌ\ÊV}iÌ>É Yes
acquired immunodeficiencies, HIV,
chronic renal failure, nephrotic
syndrome, hematologic malignancies,
organ transplant, long-term
immunosuppressive therapy (e.g.
steroids, active chemotherapy, radiation)
Pneumovax 23®
Yes
Yes
Yes, revaccinate 5
years after first dose
Yes
Yes, revaccinate 5
years after first dose
IVÕ`}Ê ]ÊV>À`Þ«>Ì iÃ]ÊiÝVÕ`}Ê Þ«iÀÌiÃÆÊaVÕ`}Ê "* ]Êi« ÞÃi>]Ê
asthma
Timing and sequential administration of pneumococcal vaccines
UÊ Ê ÃÌÀÞÊÀÊÕÜÊ ÃÌÀÞÊvÊ«iÕVVV>ÊÛ>VV>ÌÊ>`ÊLÌ Ê
vaccines are indicated, patient should receive Prevnar 13® first followed
by Pneumovax 23® at a minimum of 8 weeks later (ideally 6-12 months)
UÊvÊ«>ÌiÌÊ >ÃÊÀiViÛi`Ê*iÕÛ>ÝÊÓή and both vaccines are indicated,
the patient should receive Prevnar 13® (minimum 1 year separation)
UÊvÊ«>ÌiÌÊ >ÃÊÀiViÛi`Ê*ÀiÛ>Àʣή ≥ 8 weeks ago, and both vaccines
are indicated, the patient should receive Pneumovax 23® (minimum 8
weeks separation)
UÊvÊ«>ÌiÌÊ >ÃÊÀiViÛi`ÊLÌ ÊÛ>VViÃÊ≥ 5 years ago and revaccination
is needed with Pneumovax 23®, a second dose should be administered
(minimum 5 years apart)
UÊ*>ÌiÌÃÊÜ Ê>ÀiÊÃiÛiÀiÞÊÕV«ÀÃi`Ê­i°}°Ê /]ÊÃ`ÊÀ}>Ê
transplant) should follow institutional policy when available or consult ID
for optimal timing of vaccine administration
,iviÀiVi\Ê
*Ê,iVi`>ÌÃ\Ê7,ÊÓä£{ÆÈέÎÇ®ÆnÓÓnÓxÊ>`Ê7,ÊÓä£ÓÆÈ£­{ä®Æn£Èn£°Ê
23
3.3 Agent-specific guidelines: Vaccines
Pneumococcal vaccination
There are two types of pneumococcal vaccines that are recommended
LÞÊ *Ê}Õ`iiÃÊvÀÊ>`ÕÌÊ«>ÌiÌÃ\Ê*iÕVVV>Ê«ÞÃ>VV >À`iÊ
(Pneumovax 23®, PPV23) and Pneumococcal conjugate vaccine (Prevnar
13®, PCV13). Most patients should receive both vaccines in sequential
order, but NEVER together. See table below for indications for each vaccine.
4.1 Organism-specific guidelines: Anaerobes
Organism-specific guidelines
Anaerobes
Although anaerobic bacteria dominate the human intestinal microbiome
only a few species seem to play an important role in human infections.
Infections caused by anaerobes are often polymicrobial.
UÊÊÀ>i}>ÌÛiÊL>VÊÊBacteroides spp., Prevotella spp.,
Porphyromonas spp., Fusobacterium spp.
UÊÊÀ>i}>ÌÛiÊVVVÊÊVeillonella spp.
UÊÊÀ>«ÃÌÛiÊL>VÊÊPropionibacterium spp., Lactobacillus spp.,
Actinomyces spp., Clostridium spp.
UÊÊÀ>«ÃÌÛiÊVVVÊÊPeptostreptococcus spp. and related genera
Clinical diagnosis of anaerobic infections should be suspected in the
presence of foul smelling discharge, infection in proximity to a mucosal
surface, gas in tissues or negative aerobic cultures. Proper specimen
ViVÌÊÃÊVÀÌV>ÆÊÀiviÀÊÌÊëiViÊViVÌÊ}Õ`iiÃÊ>ÌÊ ÌÌ«\ÉÉ
www.hopkinsmedicine.org/microbiology/specimen/index.html
Treatment Notes
Metronidazole
Clindamycin
Ertapenem
Cefotetan
Pip/Tazo
Amox/Clav
Penicillin
# Patients
Hidden Content
- JHH Internal use only
.
UÊÊ-ÕÀ}V>Ê`iLÀ`iiÌÊvÊ>>iÀLVÊviVÌÃÊÃÊ«ÀÌ>ÌÊLiV>ÕÃiÊ
anaerobic organisms can cause severe tissue damage.
UÊÊ«VÉÃÕL>VÌ>Ê>`Ê `>ÞVÊ>ÀiÊVÃ`iÀi`ÊÌÊLiÊivviVÌÛiÊ
empiric therapy against Gram-positive anaerobes seen in infections
24
Propionibacterium acnes
Indications for consideration of testing for P. acnes:
UÊ -ÊÃ ÕÌÊviVÌÃ
UÊ*ÀÃÌ iÌVÊÃ Õ`iÀÊÌÊviVÌÃÊ
UÊ"Ì iÀÊ«>Ì>LiÊ`iÛViÊviVÌÃ
Diagnosis
UÊÊ ÕÌÕÀiÃÊà Õ`ÊLiÊ i`ÊvÀÊ£ä£{Ê`>ÞÃÊvÊ } ÊÃÕëVÊvÀÊP. acnes
as growth is slow
UÊÊ iVÌÊvÊÌÃÃÕiÊ>`ÊyÕ`ÊëiViÃÊvÀÊVÕÌÕÀiÊÃÊ«ÀiviÀÀi`°Ê ÊÌÊ
send swabs for culture
UÊÊÕÌ«iÊÀi«ÀiÃiÌ>ÌÛiÊëiViÃÊ­«ÀiviÀ>LÞÊήÊà Õ`ÊLiÊÃiÌÊ
for shoulder joint infections to assist in distinguishing contaminants
from pathogenic isolates — these could include synovial fluid, any
inflammatory tissue, and synovium
U Tissue specimens should also be sent for histopathology
25
4.1 Organism-specific guidelines: Anaerobes
above the diaphragm. Metronidazole is not active against
microaerophilic streptococci (e.g. S. anginosus group) and should not
be used for these infections.
UÊÊ6>VÞVÊÃÊ>ÃÊ>VÌÛiÊ>}>ÃÌÊ>ÞÊÀ>«ÃÌÛiÊ>>iÀLiÃÊ­i°}°Ê
Clostridium spp., Peptostreptococcus spp., P. acnes).
UÊÊ «ÀVÊ`ÕLiÊVÛiÀ>}iÊÜÌ ÊiÌÀ`>âiÊ ÊV>ÀL>«iiÃÊ
(Meropenem, Ertapenem) or beta-lactam/beta-lactamase inhibitors
(Ampicillin/Sulbactam, Piperacillin/Tazobactam, Amoxicillin/Clavulanic
acid) is NOT recommended given the excellent anaerobic activity of
these agents.
UÊÊB. fragilis group resistance to Clindamycin, Cefotetan, Cefoxitin, and
Moxifloxacin has increased and these agents should not be used
empirically for treatment of severe infections where B. fragilis is
suspected (e.g. intra-abdominal infections).
UÊÊÃÌÊÀiÃÃÌ>ViÊÊÌ iÊB. fragilis group is caused by beta-lactamase
production, which is screened for by the JHH micro lab.
UÊÊBacteroides thetaiotaomicron is less likely to be susceptible to
*«iÀ>VÉ/>âL>VÌ>ÆÊÌ iÀivÀi]ÊÜ iÊÌ ÃÊÀ}>ÃÊÃÊÃ>Ìi`Ê
or strongly suspected (e.g. Gram negative rods in anaerobic blood
cultures in a patient on Piperacillin/tazobactam) alternative agents
with anaerobic coverage should be used until susceptibilities are
confirmed.
UÊÊ/}iVÞViÊÃÊ>VÌÛiÊ>}>ÃÌÊ>ÊÜ`iÊëiVÌÀÕÊvÊ}À>«ÃÌÛiÊ>`Ê
gram-negative anaerobic bacteria in vitro but clinical experience with
this agent is limited.
4.2 Organism-specific guidelines: P. acnes
Treatment
UÊÊ*iVÊÊÓÎÊÊÕÌÃÊ6Ê+{Ê­«ÀiviÀÀi`®
OR
UÊ* Ê>iÀ}ÞÊ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®
NOTES
UÊÊ ÊVÃÕÌÊÀiVi`i`ÊvÀÊ>ÃÃÃÌ>ViÊÜÌ ÊV ViÊ>`Ê
duration of antibiotic therapy
UÊÊP. acnes is usually a contaminant in blood culture specimens. Draw
repeat cultures and consider clinical context before treatment
UÊÊ,>ÀiÊÀi«ÀÌÃÊvÊë>ÊviVÌÃÊ >ÛiÊLiiÊÌi`ÊvÀÊP. acnes
UÊÊÊP. acnes isolutes at JHH are susceptible to Penicillin (see anaerobic
antibiogram p. 24)
UÊÊiÌÀ`>âiÊ`iÃÊÌÊ >ÛiÊ>VÌÛÌÞÊ>}>ÃÌÊP. acnes. Tetracyclines
are not routinely tested and resistance rates are variable.
UÊÊ À>`iÀÊëiVÌÀÕÊ>}iÌÃÊÃÕV Ê>ÃÊiÀ«iiÊ>`Ê*«iÀ>VÉ
tazobactam would be expected to be active for Penicillin susceptible
isolates, but these are not first-line therapy
UÊÊ-ÕÃVi«ÌLÌÞÊ`>Ì>ÊÃ Õ`ÊLiÊÕÃi`ÊÌÊ i«Ê}Õ`iÊÌ iÀ>«iÕÌVÊ`iVÃÃ
U Consider removal of associated hardware
26
Viridans group Streptococci (alpha-hemolytic streptococci)
À>ÊVÀLÌ>ÊvÊÌ iÊÀ>ÊV>ÛÌÞÊ>`ÊÊÌÀ>VÌÆÊÃ}iÊL`ÊVÕÌÕÀiÃÊ
growing these organisms often represent contamination or transient
bacteremia
Five groups
UÊÊS. anginosus group (contains S. intermedius, anginosus, and
constellatus®\ÊÊVÞÊV>ÕÃiÊ>LÃViÃÃiÃÆÊ>ÀÌÞÊ>ÀiÊ*iVÊ
susceptible
UÊÊS. bovisÊ}ÀÕ«ÊQVÌ>ÃÊS. gallolyticus subspecies gallolyticus
(associated with colon cancer—colonoscopy mandatory, endocarditis
>ÃÊ«ÀiÃiÌÊÊÊxä¯ÊvÊV>ÃiîÊ>`ÊÃÕLëiViÃÊpasteurinus
­>ÃÃV>Ìi`ÊÜÌ Ê i«>ÌL>ÀÞÊ`Ãi>Ãi]Êi`V>À`ÌÃÊiÃÃÊV®RÆÊ
majority are Penicillin susceptible
UÊS. mitis group (contains S. mitis, oralis, gordonii, and sanguinous®\Ê
VÞÊV>ÕÃiÊL>VÌiÀi>ÊÊiÕÌÀ«iVÊ«>ÌiÌÃÊ>`Êi`V>À`ÌÃÆÊ
many have Penicillin resistance
UÊÊS. salivariusÊ}ÀÕ«\ÊiÃÃÊVÊV>ÕÃiÊvÊi`V>À`ÌÃÆÊ>ÀÌÞÊ>ÀiÊ
Penicillin susceptible
UÊÊS. mutansÊ}ÀÕ«\ÊVÊV>ÕÃiÊvÊ`iÌ>ÊV>ÀiÃÆÊÕVÊV>ÕÃiÊ
vÊi`V>À`ÌÃÆÊ>ÀÌÞÊ>ÀiÊ*iVÊÃÕÃVi«ÌLi
Beta-hemolytic Streptococci
All are susceptible to Penicillin
6>À>LiÊÀ>ÌiÃÊvÊÀiÃÃÌ>ViÊÌÊ `>ÞVÆÊ>ÃÊÌ iÊVÀL}ÞÊ
laboratory to perform susceptibility testing if you plan to use
Clindamycin or macrolides for moderate to severe infections.
While anti-staphylococcal penicillins (Oxacillin and Nafcillin) are the
agents of first choice for susceptible S. aureus infections, their activity
against streptococci is sub-optimal
} ÊÀ>ÌiÃÊvÊÀiÃÃÌ>ViÊÌÊÌiÌÀ>VÞViÃÊ>`Ê/*É-8Ê«ÀiVÕ`iÊÌ iÀÊ
empiric use for infections suspected to be caused by beta-hemolytic
streptococci
UÊÊS. pyogenesÊ­}ÀÕ«ÊÊÃÌÀi«®\Ê« >ÀÞ}ÌÃ]ÊÃÊ>`ÊÃvÌÊÌÃÃÕiÊ
viVÌÃÊVÕ`}ÊiÀÞëi>Ã]ÊViÕÌÃ]ÊiVÀÌâ}Êv>ÃVÌÃÆÊ
`>ÞVÊÀiÃÃÌ>ViÊÊ£°xx°Ó¯ÆÊ>VÀ`iÊÀiÃÃÌ>ViÊÊ{ǯ°Ê
UÊÊS. agalactiaeÊ­}ÀÕ«Ê ÊÃÌÀi«®\Êi>Ì>ÊviVÌÃ]ÊviVÌÃÊvÊÌ iÊ
vi>iÊ}iÌ>ÊÌÀ>VÌ]ÊÃÊ>`ÊÃvÌÊÌÃÃÕiÊviVÌÃ]ÊL>VÌiÀi>ÆÊ
`>ÞVÊÀiÃÃÌ>ViÊÊ£ÈÓȯÆÊ>VÀ`iÊÀiÃÃÌ>ViÊÊÇÎÓ¯°Ê
27
4.3 Organism-specific guidelines: Streptococci
Streptococci
4.3 Organism specific guidelines: Multi-drug resistant Gram-negative rods
UÊÊÀÕ«Ê Ê>`ÊÊÃÌÀi«ÌVVV\ÊviVÌÃÊÃ>ÀÊÌÊS. pyogenes and
S. agalactiaeÆÊ>ÃÃV>Ìi`ÊÜÌ ÊÕ`iÀÞ}Ê`Ãi>ÃiÃÊ­i°}°Ê`>LiÌiÃ]Ê
>}>VÞ]ÊV>À`Û>ÃVÕ>ÀÊ`Ãi>Ãi®ÆÊ `>ÞVÊÀiÃÃÌ>ViÊÊH£È¯Ê
vÊ}ÀÕ«Ê Ê>`ÊHÎίÊvÊ}ÀÕ«ÊÊÃ>ÌiÃÆÊ>VÀ`iÊÀiÃÃÌ>ViÊÊ
HÓx¯ÊvÊ}ÀÕ«Ê Ê>`ÊHÓn¯ÊvÊ}ÀÕ«ÊÊÃ>ÌiðÊ
Streptococcus pneumoniae
UÊÊ ÊV>ÕÃiÊvÊÀiëÀ>ÌÀÞÊÌÀ>VÌÊviVÌÃÊVÕ`}ÊÌÌÃÊi`>]Ê
ÃÕÃÌÃ]Ê«iÕ>ÊÛ>ÊV>ÊëÀi>`ÊvÀÊÌ iÊ>ë >ÀÞÝÆÊviVÌÃÊ
involving the CNS, bones/joints and endocarditis via hematogenous
spread
UÊÊiiÌV>Þ]ÊS. pneumoniae is in the S. mitis group of viridans group
ÃÌÀi«ÌVVVÆÊVÃiµÕiÌÞ]ÊÀ>«`ÊiVÕ>ÀÊÌiÃÌÃÊ>ÞÊÌÊLiÊ>LiÊÌÊ
distinguish S. pneumoniae and streptococci in the S. mitis group.
UÊÊ*iVÊÃÊÌ iÊ>}iÌÊvÊwÀÃÌÊV ViÊvÀÊÃiÀÕÃÊS. pneumoniae
infections when it is susceptible
UÊÊ*iVÊ>`Ê ivÌÀ>ÝiÊÃÕÃVi«ÌLÌÞÊLÀi>«ÌÃÊ>ÀiÊ`vviÀiÌÊvÀÊ
CNS and non-CNS sites
MIC breakpoints for Penicillin and Ceftriaxone against
S. pneumoniae
Antibiotic
Penicillin (oral)
Penicillin (parenteral)
Non-CNS
CNS
Ceftriaxone
Non-CNS
CNS
Susceptible
≤ 0.06
Intermediate
0.12-1
Resistant
≥2
≤2
≤ 0.06
4
≥8
≥ 0.12
≤1
≤ 0.5
2
1
≥4
≥2
UÊÊ``ÌÊvÊ6>VÞVÊÌÊ ivÌÀ>ÝiÊÃÊÌÊ`V>Ìi`ÊÊÌ iÊi«ÀVÊ
treatment of non-CNS infections caused by S. pneumoniae due to low
rates of resistance
Multi-drug resistant Gram-negative rods
Patients with infection or colonization with the resistant
organisms listed below should be placed on CONTACT
precautions (see isolation chart on p. 141)
Extended spectrum beta-lactamase (ESBL)-producing organisms
UÊÊ - ÃÊ>ÀiÊiâÞiÃÊÌ >ÌÊVviÀÊÀiÃÃÌ>ViÊÌÊ>Ê«iVÃ]Ê
cephalosporins, and Aztreonam.
UÊÊ/ iÞÊ>ÀiÊÃÌÊVÞÊÃiiÊÊK. pneumoniae and K. oxytoca,
E. coli, and P. mirabilis, and these organisms are automatically
screened by the JHH microbiology lab for the presence of ESBLs.
28
/Ài>ÌiÌ\
UÊÊiÀ«iiÊ£Ê}Ê6Ê+nÊ­ÓÊ}Ê6Ê+nÊvÀÊ -ÊviVÌîÊà Õ`ÊLiÊ
used for ALL severe infections if the organism is susceptible.
UÊÊ ÀÌ>«iiÊ£Ê}Ê6Ê+Ó{ÊV>ÊLiÊÕÃi`ÊvÀÊÕV«V>Ìi`Ê1/ÊÀÊÃvÌÊÌÃÃÕiÊ
infection with adequate source control if the organism is susceptible.
UÊÊ «ÀyÝ>VÊÀÊ/*É-8ÊV>ÊLiÊÕÃi`Ê>ÃÊ>ÌiÀ>ÌÛiÃÊÌÊ ÀÌ>«iiÊ
for uncomplicated UTI or soft tissue infection with adequate source
control if the organism is susceptible. Nitrofurantoin may also be used
for uncomplicated UTI if the organism is susceptible.
Carbapenemase-producing Enterobacteriacae (CRE)
UÊ >ÀL>«ii>ÃiÃÊ>ÀiÊiâÞiÃÊÌ >ÌÊVviÀÊÀiÃÃÌ>ViÊÌÊ>Ê«iVÃ]Ê
cephalosporins, carbapenems and Aztreonam.
UÊÊVÀL}ÞÊ>LÊÃÊÊ}iÀÊ«iÀvÀ}ÊÌ iÊ`wi`Ê`}iÊÌiÃÌ
UÊvÊV>ÀL>«iiÊÃÊÀiÃÃÌ>ÌÊÊVÀL}ÞÊ>LÊÜÊÀi«ÀÌÊÀ}>ÃÊ
>ÃʺV>ÀL>«iiÊÀiÃÃÌ>Ì»ÆÊ ÜiÛiÀ]ÊÌ iÊiÝ>VÌÊiV >ÃÊvÊ
resistance is not tested for at this time.
/Ài>ÌiÌ\Ê
UÊiÀ«iiÊÓÊ}Ê6Ê+nÊvÕÃi`ÊÛiÀÊÎÊ ÕÀÃÊà Õ`ÊLiÊVÕ`i`Ê
in most regimens based on data from small, retrospective studies
showing benefit even when the isolate is intermediate or resistant.
UÊÌÊi>ÃÌÊiÊ>``Ì>Ê>}iÌÊÃ Õ`ÊLiÊ>``i`ÊL>Ãi`ÊÊÃÕÃVi«ÌLÌiÃÊ
(e.g. Amikacin, Tigecycline, Colistin) except for UTI.
Multi-drug resistant (MDR) gram-negative organisms: defined as
organisms susceptible to NO MORE than ONE of the following antibiotic
V>ÃÃiÃ\ÊV>ÀL>«iiÃ]Ê>}ÞVÃ`iÃ]ÊyÕÀµÕiÃ]Ê«iVÃ]Ê
or cephalosporins. Note: susceptibility to sulfonamides, tetracyclines,
polymixins, and Sulbactam are NOT considered in this definition
Treatment
MDR Pseudomonas aeruginosa
MDR Acinetobacter baumannii/calcoaceticus
complex
UÊÊ ivÌâ>iÉÌ>âL>VÌ>ÊÊ
(if susceptible)
ORÊ
UÊÊÌ«ÃiÕ`>Ê-lactam PLUS
ÊÊÊ>}ÞVÃ`iÊvÊÃÞiÀ}ÞÊ«Ài`VÌi`ÊÊ
or confirmed
OR
UÊÊ ÃÌÊ­vÊÃÕÃVi«ÌLi®Ê
Ê
UÊ-lactam PLUS aminoglycoside if synergy expected
OR
UÊÊ ÃÌÊ­vÊÃÕÃVi«ÌLi®Ê
OR
UÊÊ«VÉÃÕL>VÌ>Ê­vÊÃÕÃVi«ÌLi®ÊPLUS
aminoglycoside (Sulbactam component has in vitro
activity against Acinetobacter spp.)
ÊÊÊOR
UÊÊ/}iVÞViÊ­vÊÃÕÃVi«ÌLiÆÊvÀÊviVÌÃÊÌ iÀÊÌ >Ê
bacteremia)
*Combination therapy should be considered in severe infections.
29
4.4 Organism specific guidelines: Multi-drug resistant Gram-negative rods
UÊÊ,ÃÊv>VÌÀÃÊvÀÊviVÌÊÀÊVâ>Ì\ÊÀiViÌÊ Ã«Ì>â>ÌÊ>ÌÊ>Ê
institution with a high rate of ESBLs, residence in a long-term care
facility and prolonged use of broad spectrum antibiotics.
4.4 Organism specific guidelines: Multi-drug resistant Gram-negative rods
Synergy:
UÊvÊÌ iÊÀ}>ÃÊÃÊÌiÀi`>ÌiÊÌÊ>ÊLiÌ>>VÌ>Ê>`ÊÃÕÃVi«ÌLiÊÌÊ
aminoglycosides, synergy can be assumed.
UÊ/ iÊVÀL}ÞÊ>LÊ`iÃÊÌÊ«iÀvÀÊÃÞiÀ}ÞÊÌiÃÌ}°Ê
Antibiotic doses for MDR and carbapenemase-producing
infections – normal renal and hepatic function
UÊiÀ«ii\ÊÓÊ}Ê6Ê+n]ÊvÕÃiÊÛiÀÊÎÊ ÕÀÃÊ
UÊ ivi«i\ÊÓÊ}Ê6Ê+n]ÊvÕÃiÊÛiÀÊÎÊ ÕÀÃ
UÊ ivÌ>â`iÉ ivi«i\ÊÓÊ}Ê6ÊLÕÃÊ>`}Ê`ÃiÊÛiÀÊÎäÊÕÌiÃ]Ê
then 6 g IV as continuous infusion over 24 hours
UÊ*«iÀ>VÉÌ>âL>VÌ>\ÊΰÎÇxÊ}Ê6ÊLÕÃÊ>`}Ê`ÃiÊÛiÀÊÎäÊ
minutes, then continuous infusion 3.375 g IV Q4H infused over 4
hours OR 4.5 g IV Q6H, infuse over 4 hours
UÊ ÃÌ\ÊxÊ}É}ÊVi]ÊÌ iÊÓ°xÊ}É}Ê6Ê+£ÓÊ­vÀÊ>``Ì>Ê
information, see p. 9)
UÊ«VÉÃÕL>VÌ>\ÊÎÊ}Ê6Ê+{Ê­vÀÊ ,ÊA. baumannii only)
UÊ}ÞVÃ`iÃÊ­vÀÊ`Ã}]ÊÃiiÊ«°Ê£{È®
UÊ/}iVÞVi\Ê£ää£xäÊ}Ê6Ê+£ÓÊ
UÊ ivÌâ>iÉÌ>âL>VÌ>Ê£°xÎÊ}Ê6Ê+n
,iviÀiViÃ\Ê
- ÃÊ>`ÊVV>ÊÕÌViÃ°Ê ÊviVÌÊ ÃÊÓä£x\ÊÈä­®\ʣΣ\Óx°
Current therapies for P. aeruginosa°Ê ÀÌÊ >ÀiÊ ÊÓäänÆÓ{\ÓÈ£°Ê
L>ÌÊÌ iÀ>«ÞÊvÀÊ , °Ê ÊVÀLÊviVÊÓä£{ÆÓä\ÊnÈÓÇÓ°
30
Gram-positive cocci
Gram-negative cocci
Aerobic
In clusters
UÊ >}Õ>ÃiÊ­³®\ÊS. aureus
UÊÊ >}Õ>ÃiÊ­q®\ÊS. epidermidis,
S. lugdunensis
In pairs/chains
UÊÊ «VVVÕÃ]Ê+ÕiÕ}Ê«ÃÌÛi\Ê
S. pneumoniae
UÊÊ« > iÞÌV\Ê6À`>ÃÊ}ÀÕ«ÊÊ
Streptococci, Enterococcus
(faecalis and faecium)
UÊÊ iÌ> iÞÌV\Ê
Group A strep (S. pyogenes),
Group B strep (S. agalactiae),
Group C, D, G strep
Aerobic
«VVVÕÃ\ÊN. meningiditis, N.
gonorrhoeae, Moraxella catarrhalis
VVL>VÕÃ\ H. flu, Acinetobacter spp.,
HACEK organisms
Anaerobic: Peptostreptococcus spp.
Anaerobic: Veillonella spp.
Gram-positive rods
Gram-negative rods
Aerobic
>À}i\ Bacillus spp.
VVL>VÕÃ\ÊListeria monocytogenes,
Lactobacillus spp.
->]Ê«iÀ« V\ Corynebacterium spp.
À>V }Êw>iÌÃ\ Nocardia spp.,
Streptomyces spp.
Aerobic
Lactose fermenting: Citrobacter spp.,
Enterobacter spp., E. coli, Klebsiella
spp., Serratia spp.*
Non-lactose fermenting
UÊÊ"Ý`>ÃiÊ­q®: Acinetobacter spp.,
Burkholderia spp., E. coli (rare), Proteus
spp., Salmonella spp., Shigella spp.,
Serratia spp.*, Stenotrophomonas
maltophilia
UÊÊ"Ý`>ÃiÊ ­³®\Ê P. aeruginosa, Aeromonas
spp., Vibrio spp., Campylobacter spp.
(curved)
Anaerobic
>À}i\ÊClostridium spp.
Small, pleomorphic: P. acnes, Actinomyces
spp.
Anaerobic: Bacteroides spp.,
Fusobacterium spp., Prevotella spp.
* Serratia spp. can appear initially as non-lactose fermenting due to slow fermentation.
The Johns Hopkins microbiology laboratory utilizes standard reference
methods for determining susceptibility. The majority of isolates are
tested by the automated system.
The minimum inhibitory concentration (MIC) value represents the
concentration of the antimicrobial agent required at the site of infection
for inhibition of the organism.
The MIC of each antibiotic tested against the organism is reported
with one of three interpretations S (susceptible), I (intermediate), or
R (resistant). The highest MIC which is still considered susceptible
represents the breakpoint concentration. This is the highest MIC which
is usually associated with clinical efficacy. MICs which are 1⁄ 2 q 1⁄ 8 the
31
5.1 Interpreting the microbiology report
Interpreting the microbiology report
Interpretation of preliminary microbiology data
5.1 Interpreting the microbiology report
breakpoint MIC are more frequently utilized to treat infections where
antibiotic penetration is variable or poor (endocarditis, meningitis,
osteomyelitis, pneumonia, etc.). Similarly, organisms yielding antibiotic
MICs at the breakpoint frequently possess or have acquired a low-level
resistance determinant with the potential for selection of high-level
expression and resistance. This is most notable with cephalosporins
and Enterobacter spp., Serratia spp., Morganella spp., Providencia
spp., Citrobacter spp. and Pseudomonas aeruginosa. These organisms
all possess a chromosomal beta-lactamase which frequently will be
over-expressed during therapy despite initial in vitro susceptibility. The
intermediate (I) category includes isolates with MICs that approach
attainable blood and tissue levels, but response rates may be lower than
fully susceptible isolates. Clinical efficacy can potentially be expected in
body sites where the drug is concentrated (e.g., aminoglycosides and
beta-lactams in urine) or when a higher dose of the drug can be used
(e.g., beta-lactams). The resistant (R) category indicates the organism
will not be inhibited by usually achievable systemic concentrations of the
antibiotic of normal doses.
NOTE: MIC values vary from one drug to another and from one
bacterium to another, and thus MIC values are NOT comparable
between antibiotics or between organisms.
Spectrum of antibiotic activity
The spectrum of activity table is an approximate guide of the activity of
commonly used antibiotics against frequently isolated bacteria. It takes
into consideration JHH specific resistance rates, in vitro susceptibilities
and expert opinion on clinically appropriate use of agents. For antibiotic
recommendations for specific infections refer to relevant sections of the
JHH Antibiotic Guidelines.
32
Penicillin G
Ampicillin
Ampicillin/sulbactam
Oxacillin/Nafcillin
Piperacillin/tazobactam
Cefazolin
Cefotetan
Ceftriaxone
Cefepime
Aztreonam
Ertapenem
Meropenem
Moxifloxacin
Ciprofloxacin
Azithromycin
Gent/Tobra/Amikacin
Vancomycin
Linezolid
Daptomycin
Ê
/*É-8
Clindamycin
Doxycycline
Colistin
Metronidazole
E. faecalis
Not active
GRAM-POSITIVE
E. coli
H. influenzae
Viridans strep.
S. pneumoniae
Less active or potential resistance
GRAM-NEGATIVE
Enterobacter spp.
Abdominal anaerobes
Oral anaerobes
Pseudomonas spp.
Serratia spp.
Proteus spp.
Kebsiella spp.
-hemolytic strep.
Coag. neg. staph
MSSA
MRSA
VRE
33
5.2 Spectrum of antibiotic activity
Active
Atypicals
5.3 Interpretation of rapid diagnostic tests
Interpretation of rapid diagnostic tests
The JHH microbiology lab performs rapid nucleic acid microarray testing
on blood cultures growing Gram-positive organisms and peptide nucleic
acid fluorescence in situ hybridization (PNA-FISH) testing on blood
cultures growing yeast.
Nucleic acid microarray testing (Verigine®) for Gram-positive
cocci in blood cultures
UÊÊ iÌiVÌÃÊ>`Ê`iÌwiÃÊÌ iÊÕViVÊ>V`ÃÊvÊ£ÓÊÀ>«ÃÌÛiÊL>VÌiÀ>Ê
genera/species and 3 resistance markers.
UÊÊ >VÌiÀ>ÊëiViÃ\ÊS. aureus, Coagulase-negative staphylococci, S.
lugdunensis, Staphylococcus spp. E. faecalis, E. faecium, S. pyogenes
(group A streptococci), S. agalactiae (group B streptococci), S.
pneumoniae, S. anginosus, Streptococcus spp. (e.g.,group C and G
streptococci, viridans group streptococci, etc.), Listeria spp.
UÊ,iÃÃÌ>ViÊ>ÀiÀÃ\ÊiV]ÊÛ>]ÊÛ>
Ê UÊÊvÊS. aureus is mecA positive the organism is resistant to Methicillin
and is reported as MRSA
Ê UÊÊvÊS. aureus is mecA negative the organism is susceptible to
Methicillin and is reported as MSSA
Ê UÊÊvÊ °Êfaecalis/faecium is vanA/B positive the organism is resistant
ÌÊ6>VÞVÊÊ>`ÊÃÊÀi«ÀÌi`Ê>ÃÊ6, ÆÊÌiÊÌ >ÌÊ>Ê6>VÞV
resistant E. faecalis are susceptible to Ampicillin at JHH
UÊÊ,iÃÕÌÃÊvÊÌ iÊÌiÃÌÊ>ÀiÊÀi«ÀÌi`ÊÜÌ ÊÎ{Ê ÕÀÃÊ>vÌiÀÊÌ iÊL`Ê
cultures turn positive
UÊ/iÃÌ}ÊÃÊ«iÀvÀi`ÊÞÊÊÌ iÊwÀÃÌÊ«ÃÌÛiÊL`ÊVÕÌÕÀiÊ
UÊÊ/iÃÌ}ÊÃÊ "/Ê«iÀvÀi`ÊÊL`ÊVÕÌÕÀiÃÊ}ÀÜ}ÊÀiÊÌ >ÊiÊ
Gram positive organism but is performed on blood cultures growing
both Gram positive and negative organisms
UÊÊvÊÌ iÊÌiÃÌÊÃÊi}>ÌÛiÊÌÊÜÊLiÊÀi«ÀÌi`Ê>ÃÊi}>ÌÛiÊvÀÊÌ iÊvÜ}Ê
À}>ÃÃ\Ê-Ì>« ÞVVVÕÃÊë«]ÊStreptococcus spp., E. faecalis, E.
faecium, Listeria spp.
34
Preferred empiric therapy
Alternative empiric therapy
(% susceptible in blood at JHH) if PCN allergic
MSSA Ê
"Ý>VÊ­£ä䯮Ê
ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê iv>âÊ
Ê
Ê
-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞV1
MRSAÊ
6>VÞVÊ­£ä䯮Ê
>«ÌÞV
Ê
-}iÊ«ÃÌÛiÊVÕÌÕÀiÃÊ>ÀiÊvÌiÊ>ÊVÌ>>ÌÆÊÊÌÀi>ÌiÌÊ
Coagulase-negative recommended. See p. 60 of the JHH Antibiotic Guidelines for
staphylococci
information and indications for treatment. Call the microbiology lab for
more information and further work up if infection suspected (5-6510).
"Ý>Vʭȯ®ÊÀÊ >«ÌÞVÊ
S. lugdunensisÊ
6>VÞVÊ­£ä䯮2Ê
E. faecalisÊ
«VÊ­n¯®Ê
6>VÞVÊ­x¯®1
3
E. faecium (VRE)Ê
iâ`Ê­nǯ® Ê
>«ÌÞVʭǯ®
E. faecium (not VRE)Ê6>VÞVÊ­£ä䯮3
Linezolid
4
Streptococcus spp.Ê V}ÞÊ«>ÌiÌ\Ê ivÌÀ>Ýi -iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞV1
Ê
"V}ÞÊ«>ÌiÌ\Ê6>VÞV4
S. anginosus Ê
*iVÊÊ­£ä䯮Ê
ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê ivÌÀ>Ýi
Ê
Ê
-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞV1
S. pyogenes Ê
*iVÊÊ­£ä䯮Ê
ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê iv>â
(group A strep)
-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞV1
S. agalactiae Ê
*iVÊÊ­£ä䯮Ê
ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê iv>â
(group B strep)
Ê
-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞV1
4
S. pneumoniae Ê
ivÌÀ>ÝiÊ­£ä䯮 Ê
-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞV1
(not meningitis)
S. pneumoniae Ê
ivÌÀ>ÝiʳÊ6>VÞVÊÊ
-iÛiÀiÊ* Ê>iÀ}Þ\Ê
(meningitis)
À>« iVʳÊ6>VÞV1
Listeria spp. Ê
«VÊ­£ä䯮Ê
/ÀiÌ «ÀÉÃÕv>iÌ Ý>âi
1Consult
allergy for skin testing /desensitization
to Oxacillin if found to be susceptible
to Ampicillin if found to be susceptible
4Narrow to Penicillin G if found to be susceptible
2Narrow
3Narrow
PNA-FISH for yeast
UÊÊvÊ* -ÊÃ ÜÃÊC. albicans, most non-oncology patients without
prior azole exposure can be treated with fluconazole. For more
information see p. 117 and 134.
UÊÊvÊ* -ÊÃ ÜÃÊC. glabrata, treat with Micafungin until
susceptibilities available. For more information see p. 117 and 134.
UÊÊvÊ* -Êi}>ÌÛiÊvÀÊC. albicans or C. glabrata, most cases can be
treated as unspeciated candidemia, unless cryptococcus is suspected
(send serum cryptococcal antigen). For more information see p. 117
and 134.
35
5.3 Interpretation of rapid diagnostic tests
Organism
6.1 Abdominal infections
Biliary tract infections – cholecystitis and
cholangitis
EMPIRIC TREATMENT
Community-acquired infections in patients without previous
biliary procedures AND who are not severely ill
UÊ ivÌÀ>ÝiÊ£Ê}Ê6Ê+Ó{
OR
UÊÊ ÀÌ>«iiÊ£Ê}Ê6Ê+Ó{
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê «ÀyÝ>VÊ{ääÊ}Ê6Ê+£Ó
Hospital-acquired infections OR patients with multiple therapeutic
biliary manipulations (e.g. stent placement/exchange, bilio-enteric
anastamosis of any severity) OR patients who are severely ill
UÊÊ*«iÀ>VÉÌ>âL>VÌ>ÊΰÎÇxÊ}Ê6Ê+È
OR
UÊÊ ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê ivi«iÊ£Ê}Ê6Ê+nÊPLUS Metronidazole
500 mg IV Q8H
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\ÊâÌÀi>Ê£Ê}Ê6Ê+nÊPLUS Metronidazole 500
mg IV Q8H Vancomycin (see dosing section, p. 150)
In severely ill patients with cholangitis and complicated cholecystitis,
adequate biliary drainage is crucial as antibiotics will not enter bile in
the presence of obstruction.
Duration
UÊÊUncomplicated cholecystitis\ÊÌÀi>ÌÊÞÊÕÌÊLÃÌÀÕVÌÊÃÊÀiiÛi`°Ê
NO post-procedure antibiotics are necessary if the obstruction is
successfully relieved.
UÊÊ «V>Ìi`ÊV iVÞÃÌÌÃ\Ê{Ê`>ÞÃ]ÊÕiÃÃÊ>`iµÕ>ÌiÊÃÕÀViÊVÌÀÊÃÊ
not achieved.
U Ê >ÀÞÊÃi«ÃÃ\Ê{ÇÊ`>ÞÃ]ÊÕiÃÃÊ>`iµÕ>ÌiÊÃÕÀViÊVÌÀÊÃÊÌÊ
achieved.
TREATMENT NOTES
Microbiology
UÊÊÀ>i}>ÌÛiÊÀ`ÃÊqÊE. coli, Klebsiella spp., Proteus spp.,
P. aeruginosa (mainly in patients already on broad-spectrum antibiotics
or those who have undergone prior procedures)
UÊÊ>iÀLiÃÊqÊBacteroides spp., generally in more serious infections, or
Ê«>ÌiÌÃÊÜÌ Ê>Ê ÃÌÀÞÊvÊL>ÀÞÊ>«Õ>ÌÃÆÊÀ>ÀiÊÊÕV«V>Ìi`Ê
and community-acquired infections
UÊÊEnterococcus spp°ÊqÊÌÀi>ÌiÌÊÌÊ>Ü>ÞÃÊ`V>Ìi`ÆÊÕÃiÊVV>ÊÕ`}iÌ
UÊÊ9i>ÃÌÊqÊÀ>Ài
39
6.1 Abdominal infections
Management
UÊÊÊV>ÃiÃÊvÊÕV«V>Ìi`Ê>VÕÌiÊV iVÞÃÌÌÃ]Ê>ÌLÌVÃÊÃ Õ`ÊLiÊ
given until the biliary obstruction is relieved (either by surgery, ERCP,
or percutaneous drain).
UÊÊ/Ài>ÌiÌÊvÊiÌiÀVVVÊÃÊÕÃÕ>ÞÊÌÊii`i`ÊÊ`É`iÀ>ÌiÊ
disease.
UÊÊ9i>ÃÌÊ}iiÀ>ÞÊÃ Õ`ÊLiÊÌÀi>Ìi`ÊÞÊvÊÌ iÞÊ>ÀiÊÀiVÛiÀi`ÊvÀÊ
biliary cultures, not empirically.
,iviÀiViÃ\
>ÀÞÊÌÀ>VÌÊviVÌÃ\Ê ÀÕ}ÃÊ£ÆxÇ­£®\n££°
-ÊÕ`iiÃÊvÀÊÌÀ>>L`>ÊviVÌÃ\Ê ÊviVÌÊ ÃÊÓä£äÆxä\£ÎÎq£È{°
- ÀÌÊVÕÀÃiÊÌ iÀ>«ÞÊvÀÊ\Ê Ê }ÊÊi`ÊÓä£xÆÎÇÓ\£ÈqÓääx°
Diverticulitis
EMPIRIC TREATMENT
NOTE: Patients with uncomplicated diverticulitis (defined as CT
VwÀi`ÊivÌÃ`i`Ê`Ãi>ÃiÊÜÌ ÕÌÊ>LÃViÃÃÆÊvÀiiÊ>ÀÊÀÊwÃÌÕ>ʱ fever
and elevated inflammatory markers), can be treated conservatively
without antibiotics based on a RCT.
Mild/moderate infections – can be oral if patient can take PO
UÊÊÝVÉV>ÛÕ>>ÌiÊnÇxÊ}Ê*"Ê+£Ó
OR
UÊÊ ivÌÀ>ÝiÊ£Ê}Ê6Ê+Ó{ÊPLUS Metronidazole 500 mg IV/PO Q8H
OR
UÊÊ ÀÌ>«iiÊ£Ê}Ê6Ê+Ó{
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\ÊQ «ÀyÝ>VÊ{ääÊ}Ê6Ê+£ÓÊ",Ê «ÀyÝ>VÊ
xääÊ}Ê*"Ê+£ÓRÊPLUS Metronidazole 500 mg IV/PO Q8H
Severe infections
UÊÊ*«iÀ>VÉÌ>âL>VÌ>ÊΰÎÇxÊ}Ê6Ê+È
OR
UÊÊ ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê ivi«iÊ£Ê}Ê6Ê+nÊPLUS Metronidazole
500 mg IV Q8H
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\ÊQ «ÀyÝ>VÊ{ääÊ}Ê6Ê+£ÓÊ",ÊâÌÀi>Ê
£Ê}Ê6Ê+nRÊPLUS Metronidazole 500 mg IV Q8H
Duration
UÊ{Ê`>ÞÃ]ÊÕiÃÃÊ>`iµÕ>ÌiÊÃÕÀViÊVÌÀÊÃÊÌÊ>V iÛi`°
40
Microbiology
UÊÊÃÌÊ>ÊviVÌÃÊ>ÀiÊ«ÞVÀL>
UÊÊÃÌÊVÞÊÃ>Ìi`Ê>iÀLVÊÀ}>ÃÃÊqÊE. coli, K. pneumoniae,
Enterobacter spp., Proteus spp., Enterococcus spp.
UÊÊÃÌÊVÞÊÃ>Ìi`Ê>>iÀLVÊÀ}>ÃÃÊqÊB. fragilis, Prevotella,
Peptostreptococci
Other considerations
UÊÊÌVÀL>ÊÌÀi>ÌiÌÊvÀÊ>VÕÌiÊÕV«V>Ìi`Ê`ÛiÀÌVÕÌÃÊ>ÞÊÌÊ
accelerate recovery or prevent complications/recurrence.
UÊÊ /ÊÃV>ÊÃÊ«ÀÌ>ÌÊÊ>ÃÃiÃÃ}Êii`ÊvÀÊ`À>>}iÊÊÃiÛiÀiÊ`Ãi>Ãi°ÊÊ
,iviÀiVi\
-ÊÕ`iiÃÊvÀÊÌÀ>>L`>ÊviVÌÃ\Ê ÊviVÌÊ ÃÊÓä£äÆxä\£ÎÎq£È{°
ÌLÌVÃÊÊ>VÕÌiÊÕV«V>Ìi`Ê`ÛiÀÌVÕÌÃ°Ê ÀÊÊ-ÕÀ}ÊÓä£ÓÆ\xÎÓqxΰ
- ÀÌÊVÕÀÃiÊÌ iÀ>«ÞÊvÀÊ\Ê Ê }ÊÊi`ÊÓä£xÆÎÇÓ\£ÈqÓääx°
Pancreatitis
TREATMENT
UÊÊÌLÌVÊ«À« Þ>ÝÃÊÃÊ "/Ê`V>Ìi`ÊÊ«>ÌiÌÃÊÜÌ ÊÃiÛiÀiÊ>VÕÌiÊ
pancreatitis (SAP), including those with sterile pancreatic necrosis.
UÊÌVÀL>ÊÌ iÀ>«ÞÊ >ÃÊÊivviVÌÊÊÀL`ÌÞÊ>`ÊÀÌ>ÌÞ]Ê>`Ê
prophylactic antibiotics have been associated with a change in the
spectrum of pancreatic isolates from enteric Gram-negatives to
Gram-positive organisms and fungi.
UÊÊviVÌi`Ê«>VÀi>ÌVÊiVÀÃÃÊÃÊ`iwi`ÊLÞÊ /ÊÃV>ÊÜÌ Ê}>ÃÊÊÌ iÊ
pancreas and/or percutaneous or surgical specimen with organisms
evident on gram stain or culture. Therapy should be directed based on
culture results.
UÊÊÊ«>ÌiÌÃÊ«ÀiÃiÌ}ÊÜÌ ÊÃÕëiVÌi`Ê>L`>ÊÃi«ÃÃ]ÊVÃ`iÀÊ
i«ÀVÊÌ iÀ>«Þ\
UÊÊ*«iÀ>VÌ>âL>VÌ>Ê{°xÊ}Ê6Ê+È
OR
UÊÊ ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê ivi«iÊ£Ê}Ê6Ê+nÊPLUS Metronidazole
500 mg IV Q8H
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê «ÀyÝ>VÊ{ääÊ}Ê6Ê+£ÓÊPLUS
Metronidazole 500 mg IV Q8H
41
6.1 Abdominal infections
TREATMENT NOTES
6.1 Abdominal infections
Pancreatic penetration of selected antibiotics
Good (>40%; MIC exceeded for most relevant organisms):
fluoroquinolones, carbapenems, Ceftazidime, Cefepime, Metronidazole,
Piperacillin-tazobactam
Poor (<40%): aminoglycosides, first-generation cephalosporins,
Ampicillin
Duration
For infected pancreatic necrosis, continue antibiotics for 14 days after
source control is obtained. Continuation of antibiotics beyond this time
places the patient at risk for colonization or infection with resistant
organisms and drug toxicity.
TREATMENT NOTES
UÊÊviVÌÊ`iÛi«ÃÊÊÎäqxä¯ÊvÊ«>ÌiÌÃÊÜÌ ÊiVÀÃÃÊ`VÕiÌi`ÊLÞÊ
CT scan or at the time of surgery.
UÊÊ*i>ÊV`iViÊvÊviVÌÊVVÕÀÃÊÊÌ iÊÎÀ`ÊÜiiÊvÊ`Ãi>Ãi
UÊÊ/ iÀiÊÃÊÃÕvwViÌÊiÛ`iViÊÌÊÀiVi`ÊÃiiVÌÛiÊ}ÕÌÊ
decontamination in management of pancreatitis.
,iviÀiViÃ\
>VÊvÊÕÌÌÞÊvÊ«À« Þ>VÌVÊ>ÌLÌVÃ\ÊÊ-ÕÀ}ÊÓääÇÆÓ{x\ÈÇ{°
Õ`iiÃÊvÀÊ>>}iiÌÊvÊ-*\Ê ÀÌÊ >ÀiÊi`ÊÓää{ÆÎÓ\ÓxÓ{°
Peritonitis
DEFINITIONS
Primary peritonitis is spontaneous infection of the peritoneal cavity,
ÕÃÕ>ÞÊ>ÃÃV>Ìi`ÊÜÌ ÊÛiÀÊ`Ãi>ÃiÊ>`Ê>ÃVÌiÃÊQëÌ>iÕÃÊL>VÌiÀ>Ê
«iÀÌÌÃÊ­- *®R°Ê
Secondary peritonitis is infection of the peritoneal cavity due to
spillage of organisms into the peritoneum, usually associated with GI
perforation.
Tertiary peritonitis is a recurrent infection of the peritoneal cavity
following an episode of secondary peritonitis.
Primary peritonitis/Spontaneous bacterial
peritonitis (SBP)
EMPIRIC TREATMENT
UÊÊ ivÌÀ>ÝiÊ£Ê}Ê6Ê+£Ó
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\ÊÝyÝ>VÊ{ääÊ}Ê6É*"Ê+Ó{Ê­V>Ê ÊÀÊ
Antimicrobial Stewardship to discuss regimens for patients who have
been taking fluoroquinolones for SBP prophylaxis).
42
Duration
UÊÊ/Ài>ÌÊvÀÊ5 days
PROPHYLAXIS
Cirrhotic patients with gastrointestinal hemorrhage
UÊÊ «ÀyÝ>VÊxääÊ}Ê*"Ê ÊvÀÊÇÊ`>ÞÃÊ
UÊÊ ivÌÀ>ÝiÊ£Ê}Ê6Ê+Ó{ÊV>ÊLiÊÕÃi`ÊÞÊvÊ«>ÌiÌÊÃÊ *"]ÊÌ iÊ
switch to Ciprofloxacin 500 mg PO BID once bleeding is controlled
Non-bleeding cirrhotic patients with ascites
UÊÊ/*É-8Ê£Ê -Ê*"ÊViÊ`>Þ
OR
UÊÊvÊÃÕv>Ê>iÀ}V]Ê «ÀyÝ>VÊxääÊ}Ê*"Ê`>ÞÊ
TREATMENT NOTES
Microbiology
UÊÊÀ>i}>ÌÛiÊÀ`ÃÊ­ ÌiÀL>VÌiÀ>Vi>i]Êië°ÊE. coli and K.
pneumoniae), S. pneumoniae, enterococci, and other streptococci.
UÊÊ*ÞVÀL>ÊviVÌÊà Õ`Ê«À«ÌÊÃÕëVÊvÊÊ«iÀvÀ>Ì°
Diagnostic criteria
UÊÊÓxäÊ* Ê«iÀÊ 3 of ascitic fluid.
UÊÊ*ÃÌÛiÊVÕÌÕÀiÊÜÌ ÊÊÓxäÊ* Êà Õ`Ê«À«ÌÊÀi«i>ÌÊÌ>«°ÊvÊ* ÊÊ
250 OR culture remains positive, patient should be treated.
Follow-up
UÊÊ Ã`iÀÊÀi«i>ÌÊ«>À>ViÌiÃÃÊ>vÌiÀÊ{nÊ ÕÀÃÊvÊÌ iÀ>«Þ°
UÊÊ Ã`iÀÊV >}}Ê>ÌLÌVÃÊvÊ>ÃVÌiÃÊyÕ`Ê* Ê >ÃÊÌÊ`À««i`ÊLÞÊ
Óx¯Ê>vÌiÀÊ{nÊ ÕÀÃÊ>`ÉÀÊ«>ÌiÌÊÃÊÌÊVV>ÞÊÀië`}°
Notes on prophylaxis against SBP
UÊÊÊ«>ÌiÌÃÊÜÌ ÊVÀÀ ÃÃÊ>`ÊÕ««iÀÊÊLii`ÊÃ Õ`ÊÀiViÛiÊ
«À« Þ>ÝÃÊvÀÊÇÊ`>ÞÃÊ­xä¯Ê`iÛi«Ê- *Ê>vÌiÀÊLii`®°
UÊÊ*>ÌiÌÃÊÜ Ê}iÌÊ- *ÊÃ Õ`Ê}iÌÊvi}Ê«À« Þ>ÝÃÊÌÊ«ÀiÛiÌÊvÕÌÕÀiÊ
i«Ã`iÃÊ­{äqÇä¯ÊÀÃÊvÊÀiVÕÀÀiViÊÊ£ÊÞi>À®°
UÊÊ*À« Þ>ÝÃÊÃ Õ`ÊLiÊVÃ`iÀi`ÊvÀÊÌ ÃiÊÜÌ ÊÜÊ«ÀÌiÊ
VViÌÀ>ÌÃÊÊ>ÃVÌiÃÊ­Ê£äÊ}É®ÊÀÊÕÃÕ««ÀiÃÃÊÜ iÊ
patient is in hospital.
,iviÀiViÃ\
>}ÃÃ]ÊÌÀi>ÌiÌÊ>`Ê«À« Þ>ÝÃÊvÊ- *\ÊÊi«>ÌÊÓäääÆÎÓ\£{Ó°
>>}iiÌÊvÊÛ>ÀVi>Ê iÀÀ >}iÊÊVÀÀ ÃÃ\Êi«>Ì}ÞÊÓääÇÆ{È\ÓÓqÎn°
43
6.1 Abdominal infections
UÊÊ*>ÌiÌÃÊÜÌ ÊÃiÀÕÊVÀi>ÌiÊ£Ê}É`]Ê 1 ÊÎäÊ}É`ÊÀÊÌÌ>Ê
LÀÕLÊ{Ê}É`Êà Õ`Ê>ÃÊÀiViÛiÊLÕÊ­Óx¯®Ê£°xÊ}É}ÊÊ
day 1 and 1 g/kg on day 3 (round to the nearest 12.5 g).
6.1 Abdominal infections
Secondary peritonitis/GI perforation
EMPIRIC TREATMENT
Perforation of esophagus, stomach, small bowel, colon, or
appendix
Patient mild to moderately ill
UÊÊ ÀÌ>«iiÊ£Ê}Ê6Ê+Ó{
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê «ÀyÝ>VÊ{ääÊ}Ê6Ê+£ÓÊPLUS
Metronidazole 500 mg IV Q8H
Patient severely ill or immunosuppressed
UÊÊ*«iÀ>VÉÌ>âL>VÌ>ÊΰÎÇxÊ}Ê6Ê+È
OR
UÊÊ ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê ivi«iÊ£Ê}Ê6Ê+nÊPLUS Metronidazole
500 mg IV Q8H
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊPLUS
QâÌÀi>Ê£Ê}Ê6Ê+nÊORÊ «ÀyÝ>VÊ{ääÊ}Ê6Ê+nRÊPLUS
Metronidazole 500 mg IV Q8H
Empiric antifungal therapy is generally not indicated for GI
perforation unless patient has one of the following risk factors:
Esophageal perforation, immunosuppression, prolonged antacid or
antibiotic therapy, prolonged hospitalization, persistent GI leak.
Recommendations for patients who are clinically stable and have not
ÀiViÛi`Ê«ÀÀÊ}ÌiÀÊ>âiÊÌ iÀ>«Þ\
UÊÊÕV>âiÊ{äänääÊ}Ê6É*"Ê+Ó{
Recommendations for patients who are NOT clinically stable or have
ÀiViÛi`Ê«ÀÀÊ}ÌiÀÊ>âiÊÌ iÀ>«Þ\
UÊÊV>vÕ}Ê£ääÊ}Ê6Ê+Ó{Ê
Duration of therapy for secondary peritonitis/GI perforation
Uncomplicated
Definition
ÕÀ>ÌÊ
Complicated
iwÌÊ
Duration
44
Stomach
Small Bowel
Colon
Appendix
Operated on
within
24 hours
Ó{q{nÊ ÕÀÃÊ
Operated on
within
12 hours
Ó{q{nÊ ÕÀÃÊ
Operated on
within
12 hours
Ó{q{nÊ ÕÀÃÊ
Non-necrotic or
gangrenous
appendix
Ó{Ê ÕÀÃ
>ÌiÊ«iÀ>ÌÊÀÊÊ«iÀ>ÌÆÊÀÊiVÀÌVÉ}>}ÀiÕÃÊ>««i`Ý
4 days unless adequate source control is not achieved
,iviÀiVi\
-ÊÕ`iiÃÊvÀÊÌÀ>>L`>ÊviVÌÃ\Ê ÊviVÊ ÃÊÓä£äÆxä\£ÎÎq£È{°
- ÀÌÊVÕÀÃiÊÌ iÀ>«ÞÊvÀÊ\Ê Ê }ÊÊi`ÊÓä£xÆÎÇÓ\£ÈqÓääx°
Peritonitis related to peritoneal dialysis
EMPIRIC TREATMENT
Mild to moderate illness: intraperitoneal therapy is preferred in
most cases.
Anuric patient
UÊÊ iv>âÊ£xÊ}É}ÊÊiÊL>}Ê+Ó{Ê­£Ê}ÊvÊ«>ÌiÌÊÊÈxÊ}®ÊPLUS
UÊÊiÌ>VÊÓÊ}É}ÊÊiÊL>}Ê>`}Ê`Ãi]ÊÌ iÊiÌ>VÊä°ÈÊ
mg/kg in one bag Q24H
Patient with urine output > 100 mL/day
UÊÊ ivÌ>â`iÊ£Ê}ÊÊiÊL>}Ê+Ó{
Severe illness: systemic therapy is preferred.
UÊÊ,-/Ê "- \Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®Ê6ÊPLUS ONE
vÊÌ iÊvÜ}\
QiÌ>VÊÓÊ}É}Ê6Ê",Ê ivÌ>â`iÊ£Ê}Ê6Ê",Ê «ÀyÝ>VÊ{ääÊ
}Ê6R
45
6.1 Abdominal infections
TREATMENT NOTES
UÊÊ >ÕÃ>ÌÛiÊ>}iÌÃÊvÀÊÃ>ÊLÜi]ÊV]Ê>««i`Ý\Ê>>iÀLiÃÊ­ië°Ê
B. fragilis), Enterobacteriaceae (esp. E. coli, K. pneumoniae,
Enterobacter spp., Proteus spp.®ÆÊviVÌÃÊÕÃÕ>ÞÊ«ÞVÀL>°Ê
UÊÊ*>Ì }iÃÊV>ÕÃ}ÊÌiÀÌ>ÀÞÊ«iÀÌÌÃÊ>ÀiÊÛ>À>LiÊ>`Ê>ÀiÊvÌiÊ
ÀiÃÃÌ>ÌÊÌÊÀÊÌÊVÛiÀi`ÊLÞÊÌ iÊÌ>Ê>ÌVÀL>ÊÀi}iÆÊÌ ÕÃ]Ê>Ê
change in antimicrobials is advised.
UÊÊÊV >}iÊÊ>ÌVÀL>ÃÊÌ iÀ>«ÞÊÃ Õ`ÊLiÊVÃ`iÀi`ÊÊ«>ÌiÌÃÊ
with hospital-acquired infections who are already on antimicrobials.
UÊÊ/Ài>ÌiÌÊvÊiÌiÀVVVÊÀi>ÃÊVÌÀÛiÀÃ>ÊLÕÌÊÃ Õ`ÊLiÊ
considered in critically ill or immunocompromised patients or when
they are a dominant organism in the peritoneal culture.
UÊÊ/Ài>ÌiÌÊvÊCandida spp. is generally indicated only when they are
recovered from blood or are a dominant organism in the peritoneal
culture in critically ill or immunocompromised patients.
UÊÊ*ÃÌ«iÀ>ÌÛiÊ>ÌLÌVÃÊvÀÊ>««i`VÌÃÊ>ÀiÊÕiViÃÃ>ÀÞÊÕiÃÃÊÌ iÀiÊ
is clinical evidence of peritonitis, abscess, or gangrene.
UÊÊÌLÌVÃÊ>ÀiÊ>`ÕVÌÛiÊÌÊÃÕÀViÊVÌÀ]ÊÜ V ÊÃÊ>Ê>LÃÕÌiÊ
necessity.
UÊÊ>VÊvÊÃÕÀViÊVÌÀÊÃÊ`iwi`Ê>ÃÊ}}ÊVÌ>>ÌÊ>`ÉÀÊ>Ê
undrained collection of infection.
6.1 Abdominal infections
UÊÊ / Ê "- \Ê ÃiÊ«iÀÊ`ÀÕ}ÊiÛiÃÊ>`ÉÀÊÀi>ÊvÕVÌÆÊ
consult pharmacy for recommendations for redosing and monitoring
Duration:Ê£äq£{Ê`>ÞÃ
TREATMENT NOTES
Microbiology
UÊÊÃÌÊV>ÃiÃÊV>ÕÃi`ÊLÞÊVÌ>>ÌÊvÊÌ iÊV>Ì iÌiÀ
UÊÊ ÕÌÕÀiÃÊ>ÞÊLiÊi}>ÌÛiÊÊxqÓä¯
UÊÊÀ>«ÃÌÛiÊVVVÊ­S. aureus, coagulase-negative staphylococci,
Enterococcus spp.), Gram-negative rods, yeast (much less common)
Diagnosis
UÊÊÊ«>ÌiÌÃÊÜÌ ÊÃÕëiVÌi`Ê* Ài>Ìi`Ê«iÀÌÌÃÊà Õ`Ê >ÛiÊ* ÊyÕ`Ê
sampled for cell count, differential, gram stain, culture AND amylase.
WBC > 100/mm 3ÊÜÌ ÊÊxä¯Ê* ÊÃÕ}}iÃÌÃÊviVÌ°
UÊÊ iÛ>Ìi`Ê>Þ>ÃiÊÃÕ}}iÃÌÃÊ«>VÀi>ÌÌÃÊÀÊLÜiÊ«iÀvÀ>Ì°
UÊÊÊÃÞ«Ì>ÌVÊ«>ÌiÌÃÊÜÌ ÊVÕ`ÞÊyÕ`Ê>VV«>i`ÊLÞÊ>L`>Ê
pain and/or fever, empiric treatment should be started given the high
likelihood of infection.
UÊÊÊÃÞ«Ì>ÌVÊ«>ÌiÌÃÊÜÌ ÊVi>ÀÊyÕ`]Ê>Ì iÀÊ* ÊyÕ`ÊiÝV >}i]Ê
with a dwell time of at least 2 hours, should be sampled. The decision
to start empiric therapy in these cases will depend on how sick the
patient appears.
UÊÊÊ>ÃÞ«Ì>ÌVÊ«>ÌiÌÃÊÜÌ ÊVÕ`ÞÊyÕ`]ÊÌÊÃÊÀi>Ã>LiÊÌÊ`i>ÞÊ
therapy pending the results of cell count, gram stain, and culture.
,iviÀiVi\
-* ÊÕ`iiÃÊvÀÊ*iÀÌi>Ê >ÞÃÃÀi>Ìi`ÊviVÌÃ\Ê*iÀÌÊ >ÊÌÊÓä£äÆÎä\
ÎÎÊq{Óΰ
46
Diagnosis and testing
UÊÊ >ÃiÊ`iwÌÊvÊC. difficileÊ`>ÀÀ i>\Ê«>ÃÃ>}iÊvÊ≥ 3 unformed
stools in ≤ 24 hours AND either a positive stool test for C. difficile or
colonoscopic/histopathologic finding of pseudomembranous colitis.
UÊÊ/ iÊVÀL}ÞÊ>LÊÕÃiÃÊ>ÊÀi>ÌiÊ* ,Ê>ÃÃ>ÞÊÌÊ`iÌiVÌÊÌ iÊÌÝÊ Ê
gene, the toxin responsible for CDI. Thus, patients who are colonized
with toxigenic strains will test positive even if they do not have active
infection and clinical correlation with positive test results is important.
/ iÊÃiÃÌÛÌÞÊvÊÀi>ÊÌiÊ* ,ÊÃÊÊä¯ÊV«>Ài`ÊÌÊÌÝ}iVÊ
culture.
UÊÊ Ê "/ÊÃi`ÊÃÌÊvÀÊC. difficile testing if patients do not have
diarrhea or ileus. Hard stool, fluid obtained from colonoscopy and
rectal swabs will be rejected by the microbiology lab.
UÊÊÊ«>ÌiÌÃÊÀiViÛ}Ê>Ý>ÌÛiÃ]ÊÌÊÃÊÀiVi`i`ÊÌÊ`ÃVÌÕiÊ
laxatives for 24-48 hours prior to C. difficile stool test to see if
diarrhea improves, unless the patient is clinically unstable.
UÊÊ iV>ÕÃiÊvÊi >Vi`ÊÃiÃÌÛÌÞÊvÊ* ,]Ê`Õ«V>ÌiÊÌiÃÌ}ÊÃÊÌÊ
necessary or recommended. Testing is restricted to one specimen
within 7 days. Call the Laboratory Medicine resident or faculty member
on call for those rare instances when a second specimen is required.
UÊÊ-ÌÊvÀÊC. difficile testing should be collected prior to starting
treatment for C. difficile.
UÊÊ-«iViÃÊÃ Õ`ÊLiÊ >`ÊV>ÀÀi`ÊÌÊÌ iÊ>LÊ>ÃÊÃÊ>ÃÊ«ÃÃLiÊ>vÌiÀÊ
collection. If they cannot be transported promptly, the samples should
be refrigerated.
UÊÊ Ê "/ÊÃi`ÊvÜÕ«ÊC. difficile PCR during treatment or to
document resolution of disease, as utility of the results has not been
demonstrated.
TREATMENT
UÊÊ-/"*ÊÊ / ," Ê /-Ê7
6 ,Ê*"-- °
UÊÊ"À>ÊÌ iÀ>«ÞÊÕÃÌÊLiÊÕÃi`ÊÜ iiÛiÀÊ«ÃÃLiÊ>ÃÊÌ iÊivwV>VÞÊvÊ6Ê
Metronidazole is poorly established for CDI and there is no efficacy of
IV Vancomycin for CDI.
47
6.2 Clostridium difficile infection (CDI)
Clostridium difficile infection (CDI)
6.2 Clostridium difficile infection (CDI)
Treatment depends on clinical severity
Infection severity
Clinical manifestations
Asymptomatic
carriage*
C. difficile PCR positive without diarrhea,
ileus, or colitis
Mild or moderate
C. difficile PCR positive with diarrhea but no
manifestations of severe disease
Severe
C. difficile PCR positive with diarrhea and one or more
of the following attributable to CDI:
UÊÊ7 Ê≥ 15,000
UÊÊVÀi>ÃiÊÊÃiÀÕÊVÀi>ÌiÊ> xä¯ÊvÀÊL>Ãii
Ê
Ê
Severe Complicated
Ê
Ê
Ê
Ê
Ê
Ê
Criteria as above plus one or more of the following
attributable to CDI:
UÊÞ«ÌiÃ
UÊiÕÃÊ
UÊ/ÝVÊi}>VÊÀÊ«>VÌÃÊÊ /
UÊ*iÀvÀ>Ì
UÊ ii`ÊvÀÊViVÌÞ
UÊ 1Ê>`ÃÃÊvÀÊÃiÛiÀiÊ`Ãi>Ãi
Infection severity
Treatment
ÃÞ«Ì>ÌVÊÊ
carriage
Ê Ê "/ÊÌÀi>ÌÆÊÌÀi>ÌiÌÊV>Ê«ÀÌiÊÀi>«Ã}Ê
disease
`ÊÀÊ`iÀ>ÌiÊ
UÊiÌÀ`>âiÊxääÊ}Ê*"É /Ê+nÊ
Ê
Unable to tolerate oral therapy
UÊÊiÌÀ`>âiÊxääÊ}Ê6Ê+nÊ­ÃÕL«Ì>ÆÊÃiiÊÌiÊ
at start of CDI section above)
-iÛiÀiÊ
UÊÊ6>VÞVÊÃÕÌÊ£ÓxÊ}Ê*"É /Ê+ÈÊ
-iÛiÀiÊ «V>Ìi`Ê
Ê
UÊÊ ÃÕÌÊÃÕÀ}iÀÞÊvÀÊiÛ>Õ>ÌÊvÀÊViVÌÞÊ>`Ê Ê
UÊÊ6>VÞVÊÃÕÌÊxääÊ}ÊLÞÊ /Ê+ÈÊPLUS
Metronidazole 500 mg IV Q8H†
Ê
Unable to tolerate oral therapy or complete ileus
UÊÊ6>VÞVÊxääÊ}ÊÊxääÊÊ -Ê+ÈÊ>ÃÊÀiÌiÌÊ
enema via Foley catheter in rectum + Metronidazole
500 mg IV Q8H
I£xÓx¯ÊvÊ Ã«Ì>âi`Ê«>ÌiÌÃÊ>ÀiÊVâi`ÊÜÌ C. difficile.
† Vancomycin dose can be decreased to 125 mg PO Q6H and Metronidazole can be stopped once
the patient has stabilized.
Other indications for oral Vancomycin use
UÊ ÊÀiëÃiÊÌÊÀ>ÊiÌÀ`>âiÊ>vÌiÀÊxÊ`>ÞÃÊvÊÌ iÀ>«Þ
UÊ-iV`Êi«Ã`iÊvÊÀiVÕÀÀiÌÊ`Ãi>Ãi
UÊ*>ÌiÌÃÊÜÌ ÊÃ}wV>ÌÊÃ`iÊivviVÌÃÊÌÊiÌÀ`>âi
UÊ*>ÌiÌÃÊÜ Ê>ÀiÊ«Ài}>Ì
UÊÊ Ã`iÀÊÊ«>ÌiÌÃÊÊÈxÊÞi>ÀÃÊ}ÛiÊÀi«ÀÌÃÊvÊVÀi>Ãi`ÊÀL`ÌÞÊ
from CDI.
48
Approach to patients who need to continue broad spectrum
antibiotic therapy
UÊ iÌiÀiÊÌ iÊÃ ÀÌiÃÌÊ«ÃÃLiÊVÕÀÃiÊvÊ>ÌLÌVÊÌ iÀ>«Þ°Ê
UÊÊ,i«>ViÊÌ iÊ>ÌLÌVÊÌ >ÌÊ`ÕVi`Ê ]Ê«>ÀÌVÕ>ÀÞÊVi« >ëÀÃ]Ê
Clindamycin, and fluoroquinolones.
UÊÊvÊÌ iÊ`ÕV}Ê>}iÌÊÃÊÀi«>Vi`Ê>`ÊÌ iÊ ÊÀiÃÛiÃ]ÊV«iÌiÊ>Ê
ÃÌ>`>À`Ê£ä£{Ê`>ÞÊVÕÀÃiÊvÊ ÊÌ iÀ>«ÞÆÊÌ iÀiÊÃÊÊii`ÊÌÊiÝÌi`Ê
CDI therapy until the end of the course of antibiotic therapy.
UÊÊvÊÌ iÊ`ÕV}Ê>}iÌÊV>ÌÊLiÊÃÌ««i`ÊÀÊÀi«>Vi`]ÊVÃ`iÀÊ
continuing CDI therapy until the end of the course of antibiotic therapy
­`>Ì>Ê>ÀiÊÌi`®ÆÊ ÊÌ iÀ>«ÞÊà Õ`ÊÌÊLiÊVÌÕi`ÊLiÞ`ÊÌ iÊi`Ê
of antibiotic therapy if the patient remains asymptomatic.
Recurrent disease
UÊÊ,iÃÃÌ>ViÊÌÊiÌÀ`>âiÊÀÊ6>VÞVÊ >ÃÊÌÊLiiÊ`VÕiÌi`Ê
conclusively.
UÊÊ,iVÕÀÀiÌÊ`Ãi>ÃiÊ>vÌiÀÊ>ÊV«iÌiÊVÕÀÃiÊvÊÌ iÀ>«ÞÊVVÕÀÃÊÊHÊ
Óx¯ÊvÊ«>ÌiÌðÊ,i>«ÃiÊÃÊ`ÕiÊÌÊv>ÕÀiÊÌÊiÀ>`V>ÌiÊëÀiÃÊ­È䯮Ê
ÀÊ>VµÕÃÌÊvÊ>ÊiÜÊÃÌÀ>Ê­{䯮°Ê VÕiÌÊÀiVÕÀÀiÌÊ`Ãi>ÃiÊÜÌ Ê
repeat stool testing.
UÊÊÀÃÌÊÀiVÕÀÀiViÊÃ Õ`ÊLiÊÌÀi>Ìi`ÊÌ iÊÃ>iÊ>ÃÊÌ iÊÌ>Êi«Ã`iÆÊ
severe disease should be treated with Vancomycin.
UÊÊ-iV`ÊÀiVÕÀÀiViÊÃ Õ`ÊLiÊÌÀi>Ìi`ÊÜÌ Ê6>VÞVÊÌ>«iÀÊvÜi`Ê
by pulse dosing or fecal microbiota transplant (consult GI).
UÊvÊÃiÀÕÃÊÀÊÕÌ«iÊÀiVÕÀÀiViÃ]ÊVÃÕÌÊ °
Vancomycin taper regimen
125 mg 4 times daily ×Ê£äq£{Ê`>ÞÃ
125 mg BID × 7 days
125 mg daily × 7 days
£ÓxÊ}ÊiÛiÀÞÊÓqÎÊ`>ÞÃÊvÀÊÓqnÊÜiiÃÊ­«ÕÃiÊ`Ã}®
NOTES
Management
UÊÊ-ÕÀ}V>ÊÌiÀÛiÌÊvÀÊViVÌÞÊÃ Õ`ÊLiÊVÃ`iÀi`Êi>ÀÞÊvÊÌ iÊ
patient is clinically unstable secondary to CDI.
UÊÊ/Ài>ÌiÌÊvÊ ÊÃ Õ`ÊLiÊVÌÕi`ÊÊ«>ÌiÌÃÊÜ Ê >ÛiÊ>ÊÃÕLÌÌ>Ê
colectomy with preservation of the rectum.
UÊÊÃÌÊ«>ÌiÌÃÊÜÌ ÊÃiÛiÀiÊ ÊÃ Õ`ÊÕ`iÀ}Ê>L`>Ê /ÊÌÊÀÕiÊ
out toxic megacolon or pancolitis.
49
6.2 Clostridium difficile infection (CDI)
Duration
UÊ£äq£{Ê`>ÞÃ
6.2 Clostridium difficile infection (CDI)
UÊÊ Ê "/ÊÃi`ÊvÜÕ«ÊC.difficile PCR to document resolution of
disease.
UÊÊ ÊÌÊÕÃiÊ>ÌÌÌÞÊ>}iÌð
UÊÊ-Ì«Ê«ÀÌÊ«Õ«Ê LÌÀÃÊ­**îÊÜ iiÛiÀÊ«ÃÃLiÊ>ÃÊ`>Ì>ÊÃÕ}}iÃÌÊ
PPIs increase the risk of CDI.
UÊÊ/ iÊvvi`}Ê>ÌVÀL>Ê>}iÌÃÊÃ Õ`ÊLiÊ`ÃVÌÕi`°ÊvÊ
antimicrobials are still required, it is best to avoid cephalosporins,
Clindamycin, and fluoroquinolones.
UÊÊ*À« Þ>VÌVÊÕÃiÊvÊÀ>ÊiÌÀ`>âiÊÀÊ6>VÞVÊÊ«>ÌiÌÃÊ
receiving antimicrobial therapy for treatment of underlying infection
(other than CDI) is not recommended and may increase the patient’s
risk for CDI.
Infection control
UÊÊ*>ÌiÌÃÊÜÌ Ê Êà Õ`ÊLiÊ«>Vi`ÊÊVÌ>VÌÊ«ÀiV>ÕÌÃÊ>`ÊÃ}iÊ
rooms for the duration of hospitalization.
UÊÊ1ÃiÊÃ>«Ê>`ÊÜ>ÌiÀÊÀ>Ì iÀÊÌ >Ê>V L>Ãi`Ê >`Ê}iÊÕ«ÊiÝÌ}Ê
the room of a patient with CDI.
,iviÀiViÃ\
- É -Ê ÃiÃÕÃÊÕ`iiÃÊvÀÊ \ÊviVÌÊ ÌÀÊÃ«Ê «`iÊÓä£äÆÊ
Σ\{Σq{x{°
>VÊvÊÕÌÌÞÊvÊÌÀi>Ì}Ê ÊV>ÀÀiÀÃ\ÊÊÌiÀÊi`Ê£ÓÆÊ££Ç\ÓÇÎäÓ°
iVÌÞÊÊ \ÊÊ-ÕÀ}ÊÓääÇÆÊÓ{x\ÓÈÇÇÓ°
50
UÊFor treatment of C. difficile infection, see p. 47.
UÊ >ÀivÕÞÊ>ÃÃiÃÃÊÌ iÊ«>ÌiÌÊLivÀiÊ«ÀiÃVÀL}Ê>ÌVÀL>ð
UÊÊÃÌÊviVÌÕÃÊ`>ÀÀ i>ÊÃÊÃivÌi`Ê>`ÊÞÊÀiµÕÀiÃÊÃÕ««ÀÌÛiÊ
management.
UÊÊ/Ài>ÌiÌÊÜÌ Ê>ÌLÌVÃÊÃÊÌÊÀiVi`i`ÊvÀÊÃÌÊ`
`iÀ>ÌiÊ`Ãi>ÃiÆÊÃiiÊëiVwVÊ`V>ÌÃÊÊÌ>LiÊLiÜ°
UÊÊ6À>Ê«>Ì }iÃ]ÊÃÕV Ê>ÃÊ ÀÛÀÕÃÊ>`Ê,Ì>ÛÀÕÃÊVÞÊV>ÕÃiÊ
diarrhea and do not require antibiotics.
UÊÊÌLÌVÊÕÃiÊ>ÞÊi>`ÊÌÊ>`ÛiÀÃiÊÕÌViÃÊ­i°}°Ê iÞÌVÊÕÀiVÊ
syndrome with Shiga toxin-producing E. coli).
UÊÊÌÌÌÞÊ>}iÌÃÊÃ Õ`ÊÌÊLiÊÕÃi`ÊÊ«>ÌiÌÃÊÜÌ ÊL`ÞÊ`>ÀÀ i>]Ê
fever, or elevated WBC.
Microbiology
UÊÊ ÊÛÀ>Ê«>Ì }iÃÊÊ>VÕÌiÊVÕÌÞ>VµÕÀi`Ê`>ÀÀ i>\Ê
Salmonella, Shigella, Shiga toxin-producing E. coli, Campylobacter,
C. difficile (usually with antibiotic exposure).
UÊ ÃV>Ê`>ÀÀ i>\ÊC. difficile
UÊÊ*iÀÃÃÌiÌÊ`>ÀÀ i>ÊvÊÕV«ÀÃi`Ê­ÃÌÊiÞÊV>ÕÃiÃÊÛ>ÀÞÊ
`i«i`}ÊÊÌÞ«iÊvÊÕV«ÀÃi®\ÊGiardia, Cryptosporidium,
Cyclospora, Isospora, Microsporidia, Cytomegalovirus (CMV).
Diagnosis
UÊÊ ÌÊiÛiÀÞÊ`>ÀÀ i>ÊiÃÃÊÀiµÕÀiÃÊÃÌÊVÕÌÕÀi°Ê iVÃÊÌÊÌiÃÌÊ
should be based on suspicion for specific pathogens and/or clinical
judgment of illness severity.
UÊÊ*>ÌiÌÃÊÜÌ ÊviLÀiÊ`>ÀÀ i>ÊiÃÃiÃÊÜÌ ÊVV>Êvi>ÌÕÀiÃÊvÊ
moderate to severe disease should receive empiric therapy only after
a fecal specimen is obtained for appropriate testing.
UÊÊiV>ÊëiViÃÊvÀÊ«>ÌiÌÃÊ Ã«Ì>âi`ÊvÀÊÊÎÊ`>ÞÃÊà Õ`ÊÌÊLiÊ
submitted for routine stool culture unless a high suspicion for specific
pathogen exists and/or if the patient is immunocompromised.
UÊÊÕÌ«iÊÃÌÊiÝ>>ÌÃÊvÀÊÛ>Ê>`Ê«>À>ÃÌiÃÊ­"E*®Ê>ÀiÊvÊÜÊ
yield.
UÊÊiV>ÊiÕVÞÌiÉ>VÌviÀÀÊ>ÃÃiÃÃiÌÃÊÃ Õ`ÊÌÊLiÊÕÃi`ÊÌÊ
determine the therapeutic approach.
51
6.3 Infectious diarrhea
Infectious diarrhea
6.3 Infectious diarrhea
Treatment of infectious diarrhea
Organism/Indications for treatment
Treatment
Bacteria
Campylobacter spp.
UÊâÌ ÀÞVÊxääÊ}Ê*"Ê`>ÞÊvÀÊ£qÎÊ`>ÞÃ
/Ài>ÌiÌÊÀiVi`i`ÊvÀ\
UÊ-iÛiÀiÊiÃÃ
UÊ}iÊÊÈÊÌ ÃÊÀÊÊxäÊÞi>ÀÃ
UÊÀÃÃÊL`ÊÊÃÌ
UÊ} ÊviÛiÀ
UÊ7ÀÃi}ÊÀÊÀi>«Ã}ÊÃÞ«ÌÃ
UÊ*Ài}>VÞ
UÊÕV«ÀÃi`Ê ÃÌ
E. coli (enterotoxigenic, enteropathogenic,
enteroinvasive) or empiric therapy of
traveler’s diarrhea
UÊ «ÀyÝ>VÊxääÊ}Ê*"Ê Duration:Ê£qÎÊ`>ÞÃ
Shiga toxin producing E. coli (including
E. coliÊä£xÇ\Ç®
Treatment not recommended. Antibiotic
use associated with development of
hemolytic uremic syndrome.
Non-typhoid Salmonella spp.
UÊ «ÀyÝ>VÊxääÊ}Ê*"Ê Ê
OR
UÊÊ/*É-8Ê£ÈäÉnääÊ}Ê*"Ê Ê
(if susceptible)
OR
UÊÊ ivÌÀ>ÝiÊ£Ê}Ê6Ê+Ó{
/Ài>ÌiÌÊÀiVi`i`ÊvÀ\
UÊ-iÛiÀiÊiÃÃÊÀiµÕÀ}Ê Ã«Ì>â>Ì
UÊ}iÊÊÈÊÌ ÃÊÀÊÊxäÊÞi>ÀÃ
UÊ >VÌiÀi>
UÊ*ÀiÃiViÊvÊ«ÀÃÌ iÃiÃ
UÊ6>ÛÕ>ÀÊ i>ÀÌÊ`Ãi>Ãi
UÊ-iÛiÀiÊ>Ì iÀÃViÀÃÃ
UÊ>}>VÞÊÀÊÌ iÀÊÕV«ÀÃi
Shigella spp.
Treatment always recommended even if result
returns when patient is asymptomatic.
Duration:ÊxqÇÊ`>ÞÃÆÊ£{Ê`>ÞÃÊvÀÊ
immunocompromised host
UÊÊ/*É-8Ê£ÈäÉnääÊ}ÊÊ*"Ê Ê
(if susceptible)
OR
UÊ «ÀyÝ>VÊxääÊ}Ê*"Ê Ê
Duration:ÊÎÊ`>ÞÃÆÊÇÊ`>ÞÃÊvÀÊÕ
compromised host
Vibrio parahaemolyticus
UÊ «ÀyÝ>VÊxääÊ}Ê*"Ê ÊÝÊÎÊ`>ÞÃ
Ìi\ÊÃÃV>Ìi`ÊÜÌ Êà iwà ÊVÃÕ«Ì
Treatment recommended for severe illness
Yersinia spp.
/Ài>ÌiÌÊÀiVi`i`ÊvÀ\
UÊÕV«ÀÃi`Ê ÃÌ
UÊ >VÌiÀi>
UÊ*ÃiÕ`>««i`VÌÃÊÃÞ`Ài
52
UÊÊ/*É-8Ê£ÈäÉnääÊ}Ê*"Ê ÊÝÊÎqxÊ
days (if susceptible)
OR
UÊ «ÀyÝ>VÊxääÊ}Ê*"Ê ÊÝÊÎÊ`>ÞÃ
OR
UÊÊ ÝÞVÞViÊ£ääÊ}Ê*"Ê ÊÝÊÎÊ`>ÞÃ
(not for bacteremia)
Entamoeba histolytica
Treat all (even asymptomatic)
E. dispar & E. moshkovskii infections do not
require treatment
UÊÊiÌÀ`>âiÊÇxäÊ}Ê*"Ê/ ÊÝÊxq£äÊ
days
OR
UÊÊ/`>âiÊ£Ê}Ê*"Ê+£ÓÊÝÊÎÊ`>ÞÃ
UÊÊPLUS all patients should receive
Paromomycin 500 mg PO TID x 7 days
after the course of 1st agent complete
Asymptomatic patients
UÊÊ*>ÀÞVÊxääÊ}Ê*"Ê/ ÊÝÊÇÊ`>ÞÃ
Giardia spp.
UÊÊiÌÀ`>âiÊÓxäxääÊ}Ê*"Ê/ ÊÝÊ
Çq£äÊ`>ÞÃ
OR
U Tinidazole 2 g PO once
,iviÀiViÃ\Ê
-ÊÕ`iiÃÊvÀÊ>>}iiÌÊvÊviVÌÕÃÊ >ÀÀ i>ÆÊ ÊviVÌÊ ÃÊÓää£ÆÎÓ\ÎΣqxä°
viVÌÕÃÊ`>ÀÀ i>ÊÊ`iÛi«i`Ê>`Ê`iÛi«}ÊVÕÌÀiÃ\ÊÊ Ê>ÃÌÀiÌiÀÊÓääx\Î\ÇxÇqÇÇΰ
53
6.3 Infectious diarrhea
Parasites
6.4 Helicobacter pylori infection
Helicobacter pylori infection
NOTE: CONSIDER WITHHOLDING THERAPY INITIATION UNTIL PATIENT
DISCHARGED FROM HOSPITAL UNLESS ACUTE ULCER IS PRESENT
Established indications for testing for H. pylori and treating
positive patients
UÊÊVÌÛiÊ«i«ÌVÊÕViÀÊ`Ãi>ÃiÊ­*1 ®ÊqÊ}>ÃÌÀVÊÀÊ`Õ`i>
UÊÊ wÀi`Ê ÃÌÀÞÊvÊ*1 Ê­ÌÊ«ÀiÛÕÃÞÊÌÀi>Ìi`ÊvÀÊH. pylori)
UÊÊ>ÃÌÀVÊ/ÊÞ« >Ê­ÜÊ}À>`i®
UÊÜ}ÊÀiÃiVÌÊvÊ}>ÃÌÀVÊV>ViÀÊ
UÊ>ÞÊ ÃÌÀÞÊvÊ}>ÃÌÀVÊV>ViÀÊÊ>Ê£ÃÌÊ`i}ÀiiÊÀi>ÌÛi
UÊÌÀ« VÊ}>ÃÌÀÌÃ
Other indications where testing for H. pylori and treating positive
patients can be considered: nonulcer dyspepsia, long term PPI
use, persons using NSAID/ASA, unexplained iron deficiency anemia or
vitamin B12 deficiency, family members of patients with H. pylori with
mild dyspepsia.
First-line treatment
UÊÊÝVÊ£Ê}Ê*"Ê+£ÓÊPLUS Clarithromycin 500 mg PO Q12H
PLUS Pantoprazole 40 mg PO Q12H
OR
UÊ* Ê>iÀ}Þ
UÊÊ >ÀÌ ÀÞVÊxääÊ}Ê*"Ê+£ÓÊPLUS Metronidazole 500 mg PO
Q12H PLUS Pantoprazole 40 mg PO Q12H
OR
UÊÊ/iÌÀ>VÞViÊxääÊ}Ê*"Ê+ÈÊPLUS Metronidazole 500 mg
PO Q8H PLUS Bismuth subsalicylate 525 mg PO Q6H PLUS
Pantoprazole 40 mg PO Q12H
UÊDuration:Ê£äq£{Ê`>ÞÃ
Documented recurrence of H. pylori disease
UÊvÊ«ÃÃLi]Ê>Û`Ê>ÌLÌVÃÊ«ÀiÛÕÃÞÊÕÃi`ÊÌÊÌÀi>ÌÊH. pylori
UÊÊ/iÌÀ>VÞViÊxääÊ}Ê*"Ê+ÈÊPLUS Metronidazole 500 mg PO Q8H
PLUS Bismuth subsalicylate 525 mg PO Q6H PLUS Pantoprazole 40
mg PO Q12H
UÊDuration: 14 days
TREATMENT NOTES
Diagnosis
UÊÊ**Ã]Ê2RA, Bismuth, and antibiotics with activity against H. pylori
should be withheld for at least 4 weeks prior to testing.
54
Management
UÊÊÀÃÌÊiÊÌÀi>ÌiÌÊiÀ>`V>ÌÊÀ>ÌiÃÊiÃÌ>Ìi`ÊLiÌÜiiÊxäqÇx¯°Ê
>ÕÀiÊÃÌÊvÌiÊ`ÕiÊÌÊ >ÀÌ ÀÞVÊÀiÃÃÌ>ViÊ­£äq£x¯®Ê>`ÉÀÊ
non-adherence.
UÊÊÓÀiVi«ÌÀÊ>Ì>}ÃÌÃÊ­i°}°Ê,>Ì`i®ÊV>ÊLiÊÃÕLÃÌÌÕÌi`ÊvÀÊÌ iÊ
PPI if patients are unable to tolerate PPIs or if drug interactions are a
concern.
UÊÊÝVÊPLUS Tetracycline can NOT be used together in treatment
due to low response rates.
UÊÊ ÊÌÊÃÕLÃÌÌÕÌiÊ ÝÞVÞViÉVÞViÊvÀÊ/iÌÀ>VÞViÊÀÊÊ
Azithromycin for Clarithromycin.
UÊÊÊ«>ÌiÌÃÊÜÌ Ê«ÃÌÛiÊÌiÃÌÊÀiÃÕÌÃÊi`ÃV«ÞÊÃÊ>`>ÌÀÞÊvÀÊ>}iÊ
> 45-50 years, presence of mass GI bleeding, anemia, weight loss, or
family history of gastric cancer.
UÊÊ/iÃÌÊvÊVÕÀiÊÃÊÀiVi`i`Ê> {qnÊÜiiÃÊ«ÃÌÊÌÀi>ÌiÌ°Ê
,iviÀiViÃ\
Maastricht III Consensus Report. GutÊÓääÇÆxÈ\ÇÇÓÇn£°
ACG Guidelines. Am J GastroenterolÊÓääÇÆ£äÓ\£nän£nÓx°
55
6.4 Helicobacter pylori infection
UÊÊH. pylori stool antigen is the only FDA approved test (>ä¯ÊÃiÃÌÛÌÞÊ
and specificity).
UÊ1Ài>ÊLÀi>Ì ÊÌiÃÌÊ>ÞÊLiÊ«Ì>ÊLÕÌÊÌÊVÞÊ>Û>>Li°
UÊÊ `ÃV«ÞÊPLUSÊÀ>«`ÊÕÀi>ÃiÊÌiÃÌÊ­näqx¯ÊÃiÃÌÛÌÞÆÊÓq£ää¯Ê
specificity).
UÊÊH. pylori serology does not document current infection and should not
be used for clinical diagnosis.
6.5 Gynecologic and sexually transmitted infections
Pelvic inflammatory disease
UÊVÕ`iÃÊÃ>«}ÌÃ]ÊÌÕLÛ>À>Ê>LÃViÃÃÊ>`Ê«iÛVÊ«iÀÌÌðÊ
UÊÊÀÊÌÀi>ÌiÌÊvÊ«ÃÌ«iÀ>ÌÛiÊ«iÀÌÌÃÊÀÊÜÕ`ÊviVÌ]Ê
see p. 44 and p. 105.
TREATMENT
NOTE: Avoid use of fluoroquinolones for N. gonorrhoeae due to
ÀiÃÃÌ>ViÊ­H£ä¯ÊÊ >ÌÀiÊ ÌÞ®
UÊÊ ivÌiÌ>ÊÓÊ}Ê6Ê+£ÓÊPLUS Doxycycline* 100 mg PO BID for 14
days
OR
UÊÊ ÀÌ>«iiÊ£Ê}Ê6Ê+Ó{ÊPLUS Doxycycline* 100 mg PO BID for 14
days
OR
UÊÊ* Ê>iÀ}Þ\Ê `>ÞVÊÈääääÊ}Ê6Ê+nÊPLUS Gentamicin (see
dosing section, p. 146)
Step-down therapy once patient is afebrile
UÊÊ*ÀiviÀÀi`\Ê ÝÞVÞViÊ£ääÊ}Ê*"Ê Ê´ÊQ `>ÞVÊ{xäÊ}Ê*"Ê
QID ORÊiÌÀ`>âiÊxääÊ}Ê*"Ê RÊÌÊV«iÌiÊ£{Ê`>ÞÃÊÌÌ>
*Azithromycin 1 g PO once weekly for 2 weeks can be used in the case of Doxycycline
contraindication or intolerance.
TREATMENT NOTES
Microbiology: N. gonorrhoeae, C. trachomatis, Gardnerella spp,
Ureaplasma urealyticum, anaerobes (Prevotella spp., B. fragilis), Gramnegative rods, Streptococci
Treatment of partners
UÊÊÜiÊ`>}Ãi`ÊÜÌ Ê>VÕÌiÊ* ÊÃ Õ`ÊLiÊvviÀi`Ê6ÊÌiÃÌ}°
UÊÊ>iÊ«>ÀÌiÀÃÊvÊÜiÊÜ Ê >ÛiÊ* ÊvÌiÊ>ÀiÊ>ÃÞ«Ì>ÌV°Ê
UÊÊ-iÝÊ«>ÀÌiÀÃÊ­>iÊÀÊvi>i®ÊvÊ«>ÌiÌÃÊÜ Ê >ÛiÊ* Êà Õ`Ê
be examined and treated empirically for C. trachomatis and
N. gonorrhoeae if they have had sexual contact with the patient during
the 60 days preceding onset of symptoms in the patient, regardless
of the pathogens isolated from the patient.
Endomyometritis
TREATMENT
UÊÊ->iÊ>ÃÊvÀÊ* ÊLÕÌÊÊii`ÊvÀÊ>``ÌÊvÊ ÝÞVÞViÉâÌ ÀÞV
Duration
UÊ/Ài>ÌÊÕÌÊ«>ÌiÌÊ>viLÀiÊvÀÊÓ{q{nÊ ÕÀÃ
56
TREATMENT
UÊÊiÌÀ`>âiÊ}iÊä°Çx¯]ÊiÊvÕÊ>««V>ÌÀÊ­xÊ}®ÊÌÀ>Û>}>Þ]ÊViÊ
daily for 5 days (preferred)
OR
UiÌÀ`>âiÊxääÊ}Ê*"Ê ÊvÀÊÇÊ`>ÞÃ
OR
U `>ÞVÊÎääÊ}Ê*"Ê ÊvÀÊÇÊ`>ÞÃ
TREATMENT NOTES
Microbiology: anaerobic bacteria (Prevotella spp, Mobiluncus spp.),
G. vaginalis, Ureaplasma, Mycoplasma.
UÊÊ/Ài>ÌiÌÊÃÊÀiVi`i`ÊÊ>ÊÃÞ«Ì>ÌVÊÜiÊ>`Ê } ÊÀÃÊ
asymptomatic pregnant women.
Trichomoniasis (T.vaginalis)
NOTE: Treatment of partner recommended.
TREATMENT
UÊiÌÀ`>âiÊÓÊ}Ê*"ÊViÊ
OR
UÊiÌÀ`>âiÊxääÊ}Ê*"Ê ÊvÀÊÇÊ`>ÞÃ
Uncomplicated gonococcal urethritis, cervicitis,
proctitis
TREATMENT (includes treatment for C. trachomatis):
UÊ ivÌÀ>ÝiÊÓxäÊ}ÊÊViÊPLUS Azithromycin 1 g orally (preferred)
OR
UÊÊ ivÌÀ>ÝiÊÓxäÊ}ÊÊViÊPLUS Doxycycline 100 mg PO BID for
7 days
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\ÊâÌ ÀÞVÊÓÊ}Ê*"ÊViÊ­«Àii`V>ÌiÊÜÌ Ê
antiemetic or give snack before administration)
TREATMENT NOTES
UÊ6ÊÌiÃÌ}ÊÀiVi`i`
UÊÊ/ iÊÕÃiÊvÊ ivÌÀ>ÝiÊÃÊ«ÀiviÀÀi`ÊÛiÀÊ iwÝiÊ>`Ê iv«`ÝiÊ
due to increasing MICs for oral cephalosporins.
57
6.5 Gynecologic and sexually transmitted infections
Bacterial vaginosis
6.5 Gynecologic and sexually transmitted infections
UÊÊ Õ>ÊÌ iÀ>«ÞÊÀiVi`i`ÊvÀÊN. gonorrhoeae even if C. trachomatis
is excluded.
UÊÊ-i`Ê}ÀÀ i>ÊVÕÌÕÀiÊ­ÌÊÕViVÊ>V`Ê>«wV>ÌÊÌiÃÌ®ÊvÊÞÕÊ
suspect a treatment failure.
Syphilis
SCREENING
UÊÊ-VÀii}Ê>}ÀÌ Ê>ÌÊ\Ê>ÊÌÀi«i>ëiVwVÊ>ÌL`ÞÊÌiÃÌÊ­ ®Ê
if positive, followed by RPR. A confirmatory FTA-ABS is provided if RPR
is negative.
UÊÊÊ«ÃÌÛiÊ ]Ê>Êi}>ÌÛiÊ,*,Ê>`Ê>Ê«ÃÌÛiÊ/Ê>ÞÊLiÊ`ÕiÊÌ\Ê­£®Ê
old treated syphilis (2) old untreated syphilis (3) early syphilis.
UÊÊiÌÊ ÃÌÀÞÊ>`ÊV>Ê >ÌÀiÊ ÌÞÊi>Ì Ê i«>ÀÌiÌÊ{£äÎÈ{{{nÊ
for prior history of syphilis treatment in Maryland
UÊÊvÊ«iVÊ>iÀ}V]Ê ÊVÃÕÌÃÊÃÊÀiVi`i`ÊÌÊ}Õ`iÊÌ iÀ>«Þ
Algorithm for reverse sequence syphilis screening
CIA
RPR positive
CIA positive
RPR negative
CIA negative
UÊÊ ÃÃÌiÌÊÜÌ Ê
Treponemal test that uses a different
UÊÊvÊVÕL>Ì}ÊÀÊ
syphilis infection
>Ì}iÊ­/q -ÊÀÊ/**®
primary syphilis
(past or present)
FTA-ABS positive FTA-ABS negative is suspected,
UÊÊ,iµÕÀiÃÊ ÃÌÀV>Ê ÊUÊ*ÃÃLiÊÃÞ« ÃÊÊ UÊ-Þ« ÃÊÕiÞ
treat for early
and clinical
syphilis
ÊÊÊÊviVÌÊ
UÊvÊ«>ÌiÌÊ>ÌÊ } Ê
evaluation to
ÊUÊ,iµÕÀiÃÊÊ
ÊÊÊÀÃÊvÀÊÃÞ« Ã]
determine prior
historical and
retest in one
treatment history
clinical
month
evaluation
Neurosyphilis diagnosis
UÊÊ,iµÕÀiÃÊLÌ ÊVV>Ê­iÕÀ}V>ÊÃÞ«ÌîÊ>`Ê>LÀ>ÌÀÞÊVÀÌiÀ>°Ê
UÊÊ>LÀ>ÌÀÞÊVÀÌiÀ>Ê­>ÞÊVL>ÌÊv®\ÊÃiÀ}V>ÊiÛ`iViÊvÊ
ÃÞ« Ã]Ê«ÃÌÛiÊ -Ê6 ,Ê­xä¯ÊÃiÃÌÛÌÞÆÊ } ÊëiVwVÌÞ®]Ê -Ê
«iVÞÌÃÃÊ­xÊ7 ÉÊvÊ6ÆÊ£äÓäÊ7 ÉÊvÊ6³®]Ê -Ê
elevated protein concentration (>50 mg/dl)
UÊÊÕL>ÀÊ«ÕVÌÕÀiÊ­*®Êà Õ`ÊLiÊLÌ>i`ÊÊ«>ÌiÌÃÊÜÌ Ê«ÃÌÛiÊ
serological tests for syphilis plus neurological symptoms, serological
treatment failure (lack of four-fold decline in RPR titer), evidence of
tertiary syphilis
UÊÊ Ã`iÀÊ*ÊÊ>ÃÞ«Ì>ÌVÊ6³Ê«>ÌiÌÃÊÜÌ Ê>Ê {ÊVÕÌÊ≤350
cells/ml or RPR titer ≥£\ÎÓ
58
Early syphilis (primary, secondary, and early latent syphilis within one
year after infection)
UÊÊ*iVÊÊ iâ>Ì iÊ­ V® L-A) 2.4 million units IM once
UÊÊ-iÛiÀiÊ* Ê>iÀ}iÃ\Ê ÝÞVÞViÊ£ääÊ}Ê*"Ê ÊvÀÊÓÊÜiiÃÊÊ
Note:Ê`ÕiÊÌÊVÀi>Ãi`ÊÀiÃÃÌ>ViÊ­H{x¯ÊvÊÃÌÀ>ÃÊÊ >ÌÀiÊ>ÀiÊ
resistant), Azithromycin is not recommended.
Late latent syphilis (asymptomatic infection with positive serology >1
year after infection or latent syphilis of unknown duration)
UÊÊ*iVÊÊ iâ>Ì iÊ­ V® L-A) 2.4 million units IM weekly for 3
weeks (total of 3 doses)
Neurosyphilis (can occur during any stage of syphilis)
UÊÊ*iVÊÊÎq{ÊÊÕÌÃÊ6Ê+{ÊvÀÊ£äq£{Ê`>ÞÃ
Syphilis in pregnancy
UÊÊ*iVÊÃÊÌ iÊÞÊÀiVi`i`ÊÌ iÀ>«ÞÊÊ«Ài}>ÌÊ«>ÌiÌÃÊÜÌ Ê
any kind of syphilis. Allergy consult for penicillin desensitization is
recommended.
,iviÀiViÃ\Ê
-iÝÕ>ÞÊÌÀ>ÃÌÌi`Ê`Ãi>ÃiÃÊ
ÊÌÀi>ÌiÌÊ}Õ`iiðÊ7,ÊÓä£äÉxÊ­,,£Ó®ÆÊ
£q££ä°Ê
âÌ ÀÞVÊÛÃ°Ê ÝÞVÞViÊvÀÊ* °Ê"LÃÌiÌÊÞiVÊÓääÇÆÊ££ä­£®\xÎqÈä°
Discordant Results from Reverse Sequence Syphilis Screening. MMWR 2011/60
­äx®Æ£ÎÎq£ÎÇ
59
6.5 Gynecologic and sexually transmitted infections
TREATMENT
6.6 Catheter-related bloodstream infections
Management of catheter-related
bloodstream infections (CR-BSI)
Diagnosis
UÊÊvÊÌ iÀiÊÃÊÀiÊÌ >Ê>ÊiÀÞÌ i>ÊÀÊ 9Ê«ÕÀÕiViÊ>ÌÊÌ iÊiÝÌÊ
site, the catheter is likely infected. It should be removed and replaced
at a different site.
UÊÊ7 iÊ , -ÊÃÊÃÕëiVÌi`]ÊÓqÎÊÃiÌÃÊvÊL`ÊVÕÌÕÀiÃÊà Õ`ÊLiÊ
drawn with AT LEAST one (and preferably > 1) from peripheral sites.
Blood cultures drawn through non-tunneled catheters are more likely
to yield contaminants.
UÊÊ/ iÊÕÌÌÞÊvÊVÕÌÕÀiÃÊvÊÌ iÊV>Ì iÌiÀÊÌ«ÊÌÃivÊÃÊÌÊÜiÊ`iwi`]Ê>`Ê
should ONLY be sent when there is a clinical suspicion of infection,
NOT routinely when lines are removed. They MUST be accompanied
by two sets of blood cultures obtained as detailed above.
UÊÊ/iV µÕi\Ê/ iÊiÝÌÊÃÌiÊÃ Õ`ÊLiÊVi>i`ÊÜÌ Ê>V °Ê/ iÊ
catheter should be grasped a few centimeters proximal to the exit
site. A 5 cm segment of catheter including the tip should be cut off
with sterile scissors and placed in a sterile container.
UÊÊÊÃÌ>ViÃÊÜ iÀiÊÌ iÊL`Ê>`ÊV>Ì iÌiÀÊÌ«Ê>ÀiÊVÕÌÕÀi`Ê>ÌÊÌ iÊÃ>iÊ
time and the blood cultures are negative but the catheter tip culture is
positive, antibiotics are generally not recommended, even for patients
with valvular heart disease or immunosuppression.
UÊÊ/ iÊiÝVi«ÌÊÃÊ«>ÌiÌÃÊÜ ÃiÊV>Ì iÌiÀÊÌ«ÃÊ}ÀÜÊS. aureus and
>ÛiÊi}>ÌÛiÊL`ÊVÕÌÕÀiðÊ/ iÃiÊ«>ÌiÌÃÊà Õ`ÊÀiViÛiÊxqÇÊ
days of antibiotics.
UÊÊÊ«>ÌiÌÃÊÃ Õ`ÊLiÊvÜi`ÊVÃiÞ]Ê>`ÊÀi«i>ÌÊVÕÌÕÀiÃÊÃ Õ`Ê
be sent if clinically indicated.
UÊÊ7 iÊ>ÊV>Ì iÌiÀÀi>Ìi`Ê -ÊÃÊ>ÃÃV>Ìi`ÊÜÌ ÊV>Ì iÌiÀÊ`ÞÃvÕVÌ]Ê
consider the possibility of suppurative thrombophlebitis.
EMPIRIC TREATMENT
UÊÊ6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®Ê±Ê ivi«iÊ£qÓÊ}Ê6Ê+nÊ
(use higher dose if pseudomonas suspected)
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®Ê
±ÊQ «ÀyÝ>VÊ{ääÊ}Ê6Ê+nÊ",ÊâÌÀi>ÊÓÊ}Ê6Ê+nRʱ
Tobramycin (see dosing section, p. 146)
Empiric treatment – Gram-positive cocci in clusters in 2 or more
sets of blood cultures
UÊ6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®
60
UÊ6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®Ê
Change to
UÊ"Ý>VÊÓÊ}Ê6Ê+{ÊvÊÃÕÃVi«ÌLiÊ­«ÀiviÀÀi`ÊÌÊ6>VÞV®
Duration:
UÊÎqÇÊ`>ÞÃÊvÊV>Ì iÌiÀÊÀiÛi`Ê­«ÀiviÀÀi`®
UÊ£äq£{Ê`>ÞÃÊvÊV>Ì iÌiÀÊÃ>Û>}iÊ>ÌÌi«Ì
Methicillin-susceptible Staphylococcus aureus
UÊÊ"Ý>VÊÓÊ}Ê6Ê+{ÊvÊÃÕÃVi«ÌLi
OR
UÊÊ >>« Þ>VÌVÊ* Ê>iÀ}Þ\Ê iv>âÊÓÊ}Ê6Ê+n
OR
UÊÊ>« Þ>VÌVÊ* Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®
Methicillin-resistant Staphylococcus aureus
UÊ6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®
UÊ6>VÞVÊ>iÀ}ÞÊÀÊÌiÀ>ViÊ­ÌÊÀi`Ê>ÊÃÞ`Ài®
Ê UÊ >«ÌÞVÊn£äÊ}É}Ê6Ê+ÊÓ{
OR
Ê UÊ ivÌ>ÀiÊÈääÊ}Ê6Ê+Ên
UÊ6>VÞVÊv>ÕÀi\ÊVÃÕÌÊ
TREATMENT NOTES
UÊ,iÛiÊV>Ì iÌiÀ°Ê} ÊÀi>«ÃiÊÀ>ÌiÃÊvÊV>Ì iÌiÀÊÃÊÌÊÀiÛi`°
UÊ6>VÞVÊÃÊviÀÀÊÌÊ"Ý>VÊvÀÊÌÀi>ÌiÌÊvÊ--°
UÊÊ*>ÌiÌÃÊÜÌ ÊS. aureus bacteremia should have an echocardiogram to
rule out endocarditis. Transthoracic echo is acceptable only if the study
>`iµÕ>ÌiÞÊÛiÜÃÊÌ iÊivÌÃ`i`ÊÛ>ÛiÃÆÊÃÌÊiÝ«iÀÌÃÊÀiVi`Ê/ °
UÊÊiâ`ÊÃ Õ`ÊÌÊLiÊÕÃi`ÊÀÕÌiÞÊvÀÊÌÀi>ÌiÌÊvÊS. aureus
bacteremia
UÊ ÀÌiÀ>ÊvÀÊ>Ê£{Ê`>ÞÊVÕÀÃiÊvÊÌ iÀ>«Þ
Ê UÊÊ `V>À`ÌÃÊiÝVÕ`i`ÊÜÌ Ê/ Ê­«ÀiviÀÀi`®ÆÊ } ʵÕ>ÌÞÊ// Ê>ÞÊLiÊ
adequate in select patients
Ê UÊ Ê«>Ìi`Ê«ÀÃÌ iÃiÃ
Ê UÊÊÜÕ«ÊL`ÊVÕÌÕÀiÃÊ`À>ÜÊÓ{Ê`>ÞÃÊ>vÌiÀÊÌ iÊÌ>ÊVÕÌÕÀiÃÊ>ÀiÊ
negative for S. aureus
61
6.6 Catheter-related bloodstream infections
Coagulase-negative staphylococci (CoNS)
NOTE: Single positive cultures of CoNS should NOT be treated
unless they are confirmed by follow-up cultures, the patient is
immunosuppressed and/or critically ill, or the patient has implanted
hardware. In these cases, treatment can be started but repeat cultures
should be sent PRIOR to initiation of therapy to confirm the diagnosis.
6.6 Catheter-related bloodstream infections
Ê UÊÊ/ iÊ«>ÌiÌÊ`iviÀÛiÃViÃÊÜÌ ÊÇÓÊ ÕÀÃÊvÊÌ>ÌÊvÊivviVÌÛiÊ
antistaphylococcal therapy
Ê UÊÊ/ iÊ«>ÌiÌÊ >ÃÊÊV>â}ÊÃ}ÃÊÀÊÃÞ«ÌÃÊvÊiÌ>ÃÌ>ÌVÊ
staphylococcal infection
Ê UÊ-ÕÀViÊVÌÀÊ >ÃÊLiiÊLÌ>i`
Ê UÊÊLÃiViÊvÊÌ iÀÊV`ÌÃÊÌ >ÌÊ>ÞÊ>vviVÌÊ>LÌÞÊÌÊVi>ÀÊviVÌÊ
based on clinical judgment (e.g. poorly controlled diabetes)
UÊÊÊÌ iÀÊ«>ÌiÌÃÊÃ Õ`ÊÀiViÛiÊ{ÈÊÜiiÃÊvÊÌ iÀ>«ÞÊL>Ãi`ÊÊiÝÌiÌÊ
of infection
Enterococcus faecalis
NOTE: Can be contaminants. Draw repeat cultures to confirm before
ÃÌ>ÀÌ}ÊÌÀi>ÌiÌ°Ê£ää¯ÊvÊE. faecalis blood isolates at JHH are
susceptible to Ampicillin, which should be used unless the patient has a
PCN allergy.
UÊÊ«VÊÓÊ}Ê6Ê+{Ê
OR
UÊÊ*
Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌÊ«°Ê£xä®Ê
Duration: Çq£{Ê`>ÞÃ
Enterococcus faecium
NOTE: Can be contaminants. Draw repeat cultures to confirm before
ÃÌ>ÀÌ}ÊÌÀi>ÌiÌ°Ê/ iÊ>ÀÌÞÊ­Çn¯®ÊvÊE. faecium blood isolates
at JHH are resistant to Vancomycin. If the isolate is susceptible to
Ampicillin or Vancomycin, these agents should be used preferentially at
the doses listed above for E. faecalis bacteremia.
UÊÊiâ`ÊÈääÊ}Ê6É*"Ê+£Ó
OR
UÊ >«ÌÞVÊnq£ÓÊ}É}Ê6Ê+Ó{
TREATMENT NOTES
UÊÊ Ã`iÀÊiV V>À`}À>ÊvÊÌ iÀiÊÃÊ«iÀÃÃÌiÌÊL>VÌiÀi>Ê­> 3 days)
on antibiotics.
UÊÊ/ iÊ>``ÌÊvÊiÌ>VÊ`iÃÊÌÊ>««i>ÀÊÌÊV >}iÊÕÌViÃÊÊ
CR-BSI caused by Enterococcus in the absence of endocarditis.
Gram-negative bacilli
Antibiotic selection based on organism and susceptibilities.
Duration: Çq£äÊ`>ÞÃ
62
Candida spp.
UÊ,iviÀÊÌÊ«°Ê££ÇÊvÀÊÌÀi>ÌiÌÊvÊV>``i>
CATHETER SALVAGE
UÊÊCatheter removal is STRONGLY recommended for infections with
S. aureus, yeast and Pseudomonas, as the chance of catheter salvage
is low and the risk of recurrent infection is high.
UÊÊCatheters associated with tunnel infections CANNOT be salvaged and
should be removed.
UÊÊWhen catheter salvage is attempted, systemic antibiotics should be
given through the infected line.
UÊÊÌLÌVÊÕÃi`Ê>ÃÊVÊÌ iÀ>«ÞÊÃ Õ`Ê«ÀiviÀiÌ>ÞÊ>ÌV Ê>ÌLÌVÊ
used for systemic therapy.
Antibiotic Lock Therapy (ALT)
UÊÊÌLÌVÊVÊÌ iÀ>«ÞÊV>ÊLiÊÕÃi`ÊvÀÊV>Ì iÌiÀÊÃ>Û>}iÊin addition to
systemic antibiotics when feasible.
UÊÊ >Ì iÌiÀÊÀiÛ>Êà Õ`ÊLiÊ«iÀvÀi`ÊvÊVÕÌÕÀiÃÊÀi>Ê«ÃÌÛiÊ>vÌiÀÊ
72 hours of appropriate antibiotic lock therapy
Acceptable uses:
UÊÊ->Û>}iÊvÊ}ÌiÀÊV>Ì iÌiÀÃÊÌ >ÌÊV>ÌÊLiÊÀiÛi`Ê­i°}°Ê`>ÞÃÃÊ
catheters, implantable permanent ports or central venous catheters
for chemotherapy) when there are NO systemic complications
(hemodynamic instability, tissue hypoperfusion, septic thrombosis,
infectious endocarditis or distant septic metastases) or signs of local
infection.
Unacceptable uses:
UÊÊ- ÀÌÌiÀÊÛiÕÃÊV>Ì iÌiÀÃ
UÊÊ «V>Ìi`Ê , -Ê­i°}°ÊÌÕiÊÀÊ«ÀÌ«ViÌÊviVÌ]ÊÃiÛiÀiÊ
sepsis, septic shock, endocarditis, osteomyelitis and hematogenous
seeding at other sites)
UÊ >Ì iÌiÀÊÃ>Û>}iÊÜÌ ÊS. aureus infection.
Duration:ÊÇq£{Ê`>ÞÃÊ
63
6.6 Catheter-related bloodstream infections
TREATMENT NOTES
UÊÊ >Ì iÌiÀÃÊ>ÀiÊiÃÃÊVÞÊÌ iÊÃÕÀViÊvÊÌ iÊviVÌÆÊ ÜiÛiÀ]Ê
most advocate catheter removal if the catheter is the source.
6.6 Catheter-related bloodstream infections
Standardized Concentrations of Antibiotics for ALT
Antibiotic
Heparin (optional)
6>VÞVÊxÊ}ÉÊÊä°¯Ê -Ê
iÌ>VÊxÊ}ÉÊÊä°¯Ê -Ê
äÊÀÊxäääÊÕÌÃ
ÓxääÊÕÌÃÊ
UÊÊ/ÊÃ Õ`ÊLiÊÃÌi`ÊÊÌ iÊÕiÊvÊÌ iÊV>Ì iÌiÀÊÜ iÊÌÊÊÕÃi°
UÊÊ ÜiÊÌiÃÊÃ Õ`ÊLiÊ>ÌÊÕÊvÊnq£ÓÊ ÕÀÃÊ«iÀÊ`>ÞÊ­Õ«ÊÌÊ
Ó{q{nÊ ®
UÊÊ/ÊÛÕiÊii`i`ÊÜÊÛ>ÀÞÊLÞÊÌÞ«iÊvÊV>Ì iÌiÀÊ>`Ê>Û>>LiÊÕLiÀÊ
vÊÕiðÊÊ}iiÀ>]ÊÓqxÊÊà Õ`ÊLiÊÃÕvwViÌ°
,iviÀiViÃ\
Stability and compatibility of antimicrobial lock solutions. Am J Health-Syst Pharm.
Óä£ÎÆÇä\Ó£nxÓ£n°
IDSA Guidelines for the Diagnosis and Management of Intravascular Catheter-related
viVÌÃ\ÊClin Infect Dis ÓääÆ{\£{x°
64
NOTES:
UÊÊ iÌ>>VÌ>ÃÊ>ÀiÊhighly preferable to Vancomycin if the organism is
susceptible and if the patient is not severely allergic. Strongly consider
PCN desensitization for allergic patients.
UÊÊviVÌÕÃÊ Ãi>ÃiÃÊVÃÕÌ>ÌÊÃÊ>`ÛÃi`ÊvÀÊV>ÃiÃÊvÊivÌÃ`i`Ê
infective endocarditis and prosthetic valve endocarditis, particularly in
those in which the preferred antibiotic cannot be used or in which the
organism is resistant to usual therapy.
UÊÊ/ iÀ>«iÕÌVÊÌÀ}\Ê
UÊÊ6>VÞV
UÊÊ>ÊÌÀÕ} ÊiÛi\Ê£xqÓäÊV}É
UÊÊiÌ>VÊvÀÊÀ>«ÃÌÛiÊÃÞiÀ}Þ
UÊÊ >ÞÊ`Ã}
UÊÊ>ÊÌÀÕ} ÊiÛi\Ê1 mcg/mL
UÊÊ/À>`Ì>Ê`Ã}Ê­+n®
UÊÊ>Ê«i>ÊiÛi\ÊÎq{ÊV}É
UÊÊ>ÊÌÀÕ} ÊiÛi\Ê1 mcg/mL
UÊÊ-iiÊ«°Ê£{nÊ>`Ê«°Ê£xäÊvÀÊ`iÌ>Ã
Viridans streptococci or S. bovis with PCN MIC 0.12 mcg/mL
UÊÊ*iVÊÊÎÊÊÕÌÃÊ6Ê+{ÊvÀÊ{ÊÜiiÃ
OR
UÊÊ ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê ivÌÀ>ÝiÊÓÊ}Ê6ÉÊ+Ó{ÊvÀÊ{ÊÜiiÃ
OR
UÊÊQ*iVÊÊÎÊÊÕÌÃÊ6Ê+{Ê",Ê ivÌÀ>ÝiÊÓÊ}Ê6ÉÊ+Ó{ÊvÀÊÓÊ
ÜiiÃRÊPLUS Gentamicin 3 mg/kg IV Q24H for 2 weeks
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊvÀÊ{Ê
weeks
ÀÌiÀ>ÊvÀÊÓÊÜiiÊÌÀi>ÌiÌ\
UÊÊ*>ÌiÌÊ`iÃÊÌÊ >ÛiÊV>À`>VÊÀÊiÝÌÀ>V>À`>VÊ>LÃViÃÃ
UÊÊ À Ê20 mL/min
UÊÊ*>ÌiÌÊ`iÃÊÌÊ >ÛiÊ«>Ài`ÊnÌ ÊVÀ>>ÊiÀÛiÊvÕVÌÊ
UÊÊ*>ÌiÌÊ`iÃÊÌÊ >ÛiÊAbiotrophia, Granulicatella, or Gemella spp.
Viridans streptococci or S. bovis with PCN MIC 0.12 mcg/mL
and 0.5 mcg/mL
UÊÊQ*iVÊÊ{ÊÊÕÌÃÊ6Ê+{Ê",Ê ivÌÀ>ÝiÊÓÊ}Ê6ÉÊ+Ó{ÊvÀÊ
{ÊÜiiÃRÊPLUS Gentamicin 3 mg/kg IV Q24H for the first 2 weeks of
therapy
65
6.7 Endocarditis
Treatment of native valve endocarditis
6.7 Endocarditis
OR
UÊÊ-iÛiÀiÊ*
4 weeks
Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊvÀÊ
Viridans streptococci or S. bovis with PCN MIC > 0.5 mcg/mL
and Abiotrophia defectiva, Granulicatella spp. and Gemella spp.
UÊÊ ÃÕÌÊ
TREATMENT NOTES
UÊÊÊ«>ÌiÌÃÊÜÌ ÊS. bovis biotype I endocarditis should undergo GI
work-up to rule out underlying cancer.
Staphylococcus aureus – Methicillin susceptible, native valve,
right-sided involvement only
UÊÊ"Ý>VÊÓÊ}Ê6Ê+{
UÊÊ1ÃiÊ >vVÊvÀÊ"Ý>V`ÕVi`Ê i«>ÌÌÃ
Criteria for 2-ÜiiÊÌÀi>ÌiÌ\
UÊ*>ÌiÌÊÃÊ>ÊiVÌ}Ê`ÀÕ}ÊÕÃiÀÊÜÌ Ê>ÊÌ iÀÊVÀL`ÌiÃÊ
UÊÊivÌÃ`i`Êi`V>À`ÌÃÊÃÊÀÕi`ÊÕÌÊÜÌ Ê/ Ê­«ÀiviÀÀi`®ÊÀÊ } Ê
quality TTE
UÊÊ/Ài>ÌiÌÊÃÊÜÌ Ê"Ý>VÊÀÊ >vVÊ
UÊÊ*>ÌiÌÊ`iÃÊÌÊ >ÛiÊ -Ê­ {Ê< 200)
UÊÊ*>ÌiÌÊ`iÃÊÌÊ >ÛiÊ>Ê«>Ìi`Ê«ÀÃÌ iÃÃÊ­`>ÞÃÃÊ}À>vÌ]ÊiÌV®
UÊÊ `ÊVÕÌÕÀiÃÊ>ÀiÊi}>ÌÛiÊÜÌ Ê{Ê`>ÞÃÊ>vÌiÀÊÃÌ>ÀÌ}ÊÌ iÀ>«ÞÊ
UÊÊ/ iÀiÊÃÊÊiÛ`iViÊvÊiLVÊ`Ãi>ÃiÊ"/ ,ÊÌ >ÊÃi«ÌVÊ
pulmonary emboli
UÊÊ6i}iÌ>ÌÃÊ>ÀiÊ>Ê< 2 cm in size
UÊÊvÊ«>ÌiÌÊ`iÃÊÌÊiiÌÊVÀÌiÀ>ÊvÀÊÓÜiiÊÌÀi>ÌiÌ]ÊÌÀi>ÌÊvÀÊ{Ê
weeks
Staphylococcus aureus – Methicillin susceptible, native valve,
left-sided involvement
UÊÊ"Ý>VÊÓÊ}Ê6Ê+{Ê
OR
UÊÊ ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê iv>âÊÓÊ}Ê6Ê+nÊ
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê-ÌÀ}ÞÊVÃ`iÀÊ* Ê`iÃiÃÌâ>ÌÊÀÊ
Vancomycin (see dosing section, p. 150)
UÊÊ/ iÊ>``ÌÊvÊiÌ>VÊÌÊ>ÊLiÌ>>VÌ>Ê>ÞÊ i«ÊVi>ÀÊL`ÊVÕÌÕÀiÃÊ
faster but does not appear to affect mortality. It particularly should be
avoided in the elderly and in those with baseline renal impairment.
Staphylococcus aureus – Methicillin resistant, native valve
UÊÊ6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®
66
S. pneumoniae, and Group A streptococci
UÊÊ*iVÊÊÎÊÊÕÌÃÊ6Ê+{ÊvÀÊ{ÊÜiiÃ
OR
UÊÊ ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê ivÌÀ>ÝiÊÓÊ}Ê6Ê+Ó{ÊvÀÊ{ÊÜiiÃÊ",Ê
Cefazolin 2 g IV Q8H for 4 weeks
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊvÀÊ{Ê
weeks
UÊÊÀÊS. pneumoniae, if PCN MIC ≥ 0.1, consult ID
Groups B, C and G streptococci
UÊÊ*iVÊÊÎÊÊÕÌÃÊ6Ê+{ÊvÀÊ{qÈÊÜiiÃÊ´ÊiÌ>VÊ
3 mg/kg IV Q24H for the first 2 weeks of therapy
OR
UÊÊ ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê iv>âÊÓÊ}Ê6Ê+nÊvÀÊ{qÈÊÜiiÃʱ
Gentamicin 3 mg/kg IV Q24H for the first 2 weeks of therapy
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£{È®ÊvÀÊ{qÈÊ
weeks ± Gentamicin 3 mg/kg IV Q24H for the first 2 weeks of therapy
UÊÊ Ã`iÀÊ>Ê Ê ÃÕÌ
Enterococcus faecalis
UÊÊ«VÊ>`ÊiÌ>VÊÃÕÃVi«ÌLi\Ê«VÊÓÊ}Ê6Ê+{Ê",Ê
Penicillin G 4 million units IV Q4H PLUS Gentamicin 1 mg/kg IV Q8H
BOTH for 4-6 weeks
UÊÊ«VÊÃÕÃVi«ÌLiÊÜÌ ÊVÌÀ>`V>ÌÃÊvÀÊ>}ÞVÃ`iÃÊÀÊ
iÌ>VÊÀiÃÃÌ>Ì\Ê«VÊÓÊ}Ê6Ê+{Ê",Ê*iVÊÊ{ÊÊ
units IV Q4H PLUS Ceftriaxone 2 g IV Q12H BOTH for 4-6 weeks
67
6.7 Endocarditis
Duration
UÊÊ1V«V>Ìi`\ÊÈÊÜiiÃ
UÊÊ «V>Ìi`Ê­«iÀÛ>ÛÕ>ÀÊ>LÃViÃÃÊvÀ>Ì]ÊiÌ>ÃÌ>ÌVÊV«V>Ì]Ê
«ÀÊVÌÀi`Ê`>LiÌiÃÊiÌÕî\ÊÈÊÀÊÀiÊÜiiÃÊL>Ãi`ÊÊVV>ÊÊ
picture and response to therapy
UÊÊ Ê>`ÊV>À`>VÊÃÕÀ}iÀÞÊVÃÕÌÃÊÀiVi`i`ÊvÀÊV«V>Ìi`Ê
diseases
6.7 Endocarditis
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê-ÌÀ}ÞÊVÃ`iÀÊ* Ê`iÃiÃÌâ>ÌÊvÊ* Ê
allergy is anaphylactic or Vancomycin (see dosing section, p. 146)
PLUS Gentamicin 1 mg/kg IV Q8H BOTHÊvÀÊ{qÈÊÜiiÃ
UÊÊ/Ài>ÌÊvÀÊ{ÊÜiiÃÊÞÊÜ iÊÃÞ«ÌÃÊ >ÛiÊLiiÊ«ÀiÃiÌÊvÀÊ< 3
months AND there is a prompt response to therapy
Enterococcus faecium
UÊ ÃÕÌÊ
,iviÀiVi\
1ÃiÊvÊ ivÌÀ>ÝiÊÊiÌiÀVVV>Êi`V>À`ÌÃ\Ê ÊviVÌÊ ÃÊÓä£ÎÆÊxÈ\£ÓÈ£n°
HACEK organisms (Haemophilus parainfluenzae, H. aphrophilus,
Actinobacillus actinomycetemcomitans, Cardiobacterium
hominus, Eikenella corrodens, Kingella kingae)
UÊÊ ivÌÀ>ÝiÊÓÊ}Ê6ÉÊ+Ó{ÊvÀÊ{ÊÜiiÃ
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê ÃÕÌÊ
Gram-negative organisms, culture negative endocarditis, or
fungal endocarditis
UÊÊ ÃÕÌÊ
Treatment of prosthetic valve endocarditis
UÊÊiiÀ>ÞÊV>ÕÃi`ÊLÞÊÃÌ>« ÞVVVÊÊÌ iÊwÀÃÌÊ£qÓÊÞi>ÀÃÊvÜ}ÊÛ>ÛiÊ
replacement (both S. aureus and coagulase-negative staph). Etiologies
are similar to native valve infections 2 or more years post-op.
UÊi`V>ÊÌÀi>ÌiÌÊ>iÊÃÊvÌiÊ "/ÊivviVÌÛi°
UÊÊ«>ÌiÌÃÊÃ Õ`Ê >ÛiÊ>Ê/ °
EMPIRIC TREATMENT
UÊÊ6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊPLUS Gentamicin 1 mg/kg
IV Q8H
Viridans streptococci or S. bovis with PCN MIC 0.12 mcg/mL
UÊÊQ*iVÊÊ{ÊÊÕÌÃÊ6Ê+{Ê",Ê ivÌÀ>ÝiÊÓÊ}Ê6ÉÊ+Ó{RÊvÀÊ
6 weeks Gentamicin 3 mg/kg IV Q24H for first 2 weeks of therapy
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊvÀÊÈÊ
weeks
68
Staphylococcus aureus—Methicillin susceptible
UÊÊ"Ý>VÊÓÊ}Ê6Ê+{ÊvÀÊÈÊÜiiÃÊPLUS Gentamicin 1 mg/kg IV Q8H for
first 2 weeks of therapy
AND
UÊÊ,v>«ÊÎääÊ}Ê*"Ê+nÊvÀÊÈÊÜiiÃÊafter blood cultures have
cleared
UÊÊ Ê>`ÊV>À`>VÊÃÕÀ}iÀÞÊVÃÕÌÃÊÀiVi`i`
Staphylococcus aureus—Methicillin resistant or Coagulasenegative staphylococci
UÊÊ6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊvÀÊÈÊÜiiÃÊPLUS
Gentamicin 1 mg/kg IV Q8H for the first 2 weeks of therapy
AND
UÊÊ,v>«ÊÎääÊ}Ê*"Ê+nÊvÀÊÈÊÜiiÃÊafter blood cultures have
cleared
UÊÊvÊV>}Õ>Ãii}>ÌÛiÊÃÌ>« ÞVVVÊÃÊÃÕÃVi«ÌLiÊÌÊ"Ý>VÊÌ iÊ
treat as S. aureusÊqÊiÌ VÊÃÕÃVi«ÌLi°
UÊÊ Ê>`ÊV>À`>VÊÃÕÀ}iÀÞÊVÃÕÌÃÊÀiVi`i`
Gram-negative organisms or culture negative endocarditis
UÊÊ ÃÕÌÊ
DUKE CRITERIA FOR INFECTIVE ENDOCARDITIS
Diagnostic criteria (Modified Duke criteria)
Definite endocarditis
UÊÊ*ÀiÃiViÊvÊÓÊ>ÀÊVÀÌiÀ>Ê",Ê£Ê>ÀÊ ÊÎÊÀÊ",ÊxÊÀ
Possible endocarditis
UÊÊ*ÀiÃiViÊvÊ£Ê>ÀÊ Ê£ÊÀÊ",ÊÎÊÀÊVÀÌiÀ>
Rejected endocarditis
UÊÊÀÊ>ÌiÀ>ÌiÊ`>}ÃÃÊÌ >ÌÊiÝ«>ÃÊÊ>viÃÌ>ÌÃÊvÊ
(NOTE: simply having another infection does NOT exclude
endocarditis)
69
6.7 Endocarditis
Viridans streptococci or S. bovis with PCN MIC 0.12 mcg/mL
UÊÊQ*iVÊÊ{ÊÊÕÌÃÊ6Ê+{Ê",Ê ivÌÀ>ÝiÊÓÊ}Ê6ÉÊ+Ó{RÊ
PLUS Gentamicin 3 mg/kg IV Q24H for 6 weeks
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊvÀÊÈÊ
weeks
6.7 Endocarditis
Major criteria
Microbiologic
UÊÊ/ÜÊÃi«>À>ÌiÊL`ÊVÕÌÕÀiÃÊ«ÃÌÛiÊvÀÊ>ÊÌÞ«V>ÊÀ}>Ã\Ê
viridans streptococci, S. bovis, HACEK, S. aureus, Enterococcus
spp.
UÊÊ*iÀÃÃÌiÌÊL>VÌiÀi>ÊÜÌ Ê>ÞÊÀ}>ÃÊ>ÃÊiÛ`iVi`ÊLÞ\ÊÓÊ
positive blood cultures drawn at least 12 hours apart OR 3/3
positive blood cultures with at least 1 hour between the first and
last OR the majority of more than 4 cultures positive from any time
period.
UÊÊ*ÃÌÛiÊCoxiella burnetti (Q fever) culture or serology.
Echocardiographic (TEE strongly recommended for prosthetic valve)
UÊÊ6i}iÌ>ÌÊ­ÊÛ>ÛiÊÀÊÃÕ««ÀÌ}ÊÃÌÀÕVÌÕÀiÊ",ÊÊ«>Ì ÊvÊ
regurgitant jet)
UÊÊLÃViÃÃ
UÊÊ iÜÊ`i ÃViViÊvÊ«ÀÃÌ iÌVÊÛ>Ûi
Physical exam
UÊÊ 7ÊÀi}ÕÀ}Ì>ÌÊÕÀÕÀÊ­ÜÀÃi}ÊvÊ`ÊÕÀÕÀÊÃÊ "/Ê
sufficient)
Minor criteria
UÊÊ*Ài`ëÃ}ÊV`Ì\Ê«ÀiÛÕÃÊi`V>À`ÌÃ]ÊiVÌÊ`ÀÕ}ÊÕÃi]Ê
prosthetic valve, ventricular septal defect, coarctation of the aorta,
calcified valve, patent ductus, mitral valve prolapse with regurgitation,
IHSS or other valvular heart disease
UÊÊiÛiÀÊ≥ 38.0°C (100.4°F)
UÊÊ LVÊiÛiÌÃ\Ê>ÀÌiÀ>ÊÀÊ«Õ>ÀÞÊiL]ÊVÕVÌÛ>Ê
hemorrhage, retinal hemorrhage, splinter hemorrhage, intracranial
hemorrhage, mycotic aneurysm
UÊÊÕ}VÊ« ii\Ê"ÃiÀÊ`iÃ]Ê}iÀÕi« ÀÌÃ]Ê«ÃÌÛiÊ
rheumatoid factor
UÊÊ*ÃÌÛiÊL`ÊVÕÌÕÀiÃÊÌ >ÌÊ`½ÌÊiiÌÊVÀÌiÀ>Ê>LÛiÊ",ÊÃiÀ}VÊ
evidence of active infection with an organism known to cause
endocarditis BUT single positive cultures for coagulase-negative
staphylococci are NOT considered even a minor criterion
,iviÀiViÃ\
"À>ÊÌ iÀ>«Þ\ÊÊÊi`Ê£ÈÆÊ£ä£\ÈnÇÈ°
- ÀÌÊVÕÀÃiÊÌ iÀ>«Þ\ÊÊÌiÀÊi`Ê£{ÆÊ£Ó£\nÇÎÈ°
ÕiÊVÀÌiÀ>\Ê ÊviVÌÊ ÃÊÓäääÆÊÎä\ÈÎÎn°
Ê-ViÌwVÊ-Ì>ÌiiÌÊÊviVÌÛiÊ `V>À`ÌÃ\Ê ÀVÕ>ÌÊÓääxÆÊ£££­Óή\iÎ{{Î{°
TEE in S. aureusÊL>VÌiÀi>\ÊÊÊ Ê >À`Ê£ÇÆÊÎä\Ê£äÇÓn°
,-ÊL>VÌiÀi>Éi`V>À`ÌÃÊÀiVi`>ÌÃ\Ê ÊviVÌÊ ÃÊÓ䣣ÆÊxÓ\i£nxx
70
NOTE: Obtain at least 2 sets of blood cultures before initiation of
antibiotic therapy
EMPIRIC TREATMENT
UÊ6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®°Ê >ÀÀÜÊÌ iÀ>«ÞÊL>Ãi`ÊÊ
culture results.
TREATMENT NOTES
MicrobiologypÃÌ>« ÞVVVÊÊÇänä¯ÊvÊV>ÃiÃÊ­Hxä¯ÊV>}Õ>Ãi
i}>ÌÛiÊÃÌ>« ÞVVVÊ>`ÊHxä¯ÊS. aureus)
Management
UÊvÊL`ÊVÕÌÕÀiÃÊ>ÀiÊ«ÃÌÛiÊÀÊi`V>À`ÌÃÊÃÊÃÕëiVÌi`Ê«>ÌiÌÃÊ
should undergo transesophageal echocardiography (TEE)
UÊ «iÌiÊiÝÌÀ>VÌÊÀiVi`i`ÊvÀÊ«>ÌiÌÃÊÜÌ Ê«ViÌÊviVÌÊ
and/or valvular or lead endocarditis
UÊÌÊÌ iÊÌiÊvÊiÝÌÀ>VÌ]ÊÌÃÃÕiÊ­À>Ì iÀÊÌ >ÊÃÜ>LîÊvÀÊÌ iÊ}iiÀ>ÌÀÊ
pocket should be sent for Gram-stain and culture and lead tips should
be sent for culture.
UÊ ÌiÊÌ >ÌÊLiV>ÕÃiÊi>`ÃÊ>ÀiÊiÝÌÀ>VÌi`ÊÌ ÀÕ} Ê>Ê«iÊ}iiÀ>ÌÀÊ
«ViÌ]ÊÌ iÞÊ>ÞÊLiViÊVÌ>>Ìi`ÊLÞÊÌ iÊviVÌi`Ê«ViÌÆÊ
therefore, positive lead cultures are not always indicative of lead
endocarditis in patient with negative blood cultures.
UÊ `ÊVÕÌÕÀiÃÊÃ Õ`ÊLiÊLÌ>i`Ê>vÌiÀÊ`iÛViÊÀiÛ>°
UÊ iÛViÊÀi«>Ì>ÌÊÃ Õ`ÊLiÊÊÌ iÊVÌÀ>>ÌiÀ>ÊÃ`iÊÜ iiÛiÀÊ
possible.
UÊ «iÌiÊiÝÌÀ>VÌÊÃÊÃÌÀ}ÞÊÀiVi`i`ÊÊ>Ê«>ÌiÌÃÊ
presenting with S. aureus bacteremia and no other source
UÊ «iÌiÊiÝÌÀ>VÌÊÃ Õ`ÊLiÊVÃ`iÀi`ÊÊ«>ÌiÌÃÊÜÌ Ê«iÀÃÃÌiÌÊ
positive blood cultures with other organisms (e.g. coagulase-negative
staphylococci, enterococci, Gram-negative bacilli) on a case-by-case
basis.
UÊ «iÌiÊ`iÛViÊ>`Êi>`ÊÀiÛ>ÊÃÊÀiVi`i`ÊvÀÊ«>ÌiÌÃÊÜÌ Ê
valvular endocarditis.
UÊÌVÀL>Ê«À« Þ>ÝÃÊÃÊ "/ÊÀiVi`i`ÊvÀÊ`iÌ>ÊÀÊÌ iÀÊ
invasive procedures following placement
,iviÀiVi\Ê
Ê-ViÌwVÊ-Ì>ÌiiÌÊÊ**Ê>`Ê
ÊviVÌÃ\Ê ÀVÕ>ÌÊÓä£äÆÊ£Ó£\{xnq{ÇÇ°
71
6.8 Pacemaker/ICD infections
Permanent pacemaker (PPM) and implantable
cardioverter-defibrillator (ICD) infections
6.8 Pacemaker/ICD infections
Reimplantation timing and duration of therapy
Diagnosis
Pocket site infection
Timing of reimplantation
Blood cultures negative for
72 hours and surgical site
healing
Positive blood cultures
with rapid clearance
AND TEE with either
no vegetation or
uncomplicated lead
vegetation
Sustained positive blood
cultures AND TEE with
no vegetation or
uncomplicated lead
vegetation
Valve endocarditis
Post-explantation blood
cultures negative for
72 hours
Duration of therapy
7-10 days if device erosion
without inflammation
10-14 days all others
Oral therapy can be
considered
Non-S. aureus\ÊÓÊÜiiÃÊ
IV therapy
S. aureus\Ê{ÊÜiiÃÊ
IV therapy
Post-explantation blood
cultures negative for
72 hours
4 weeks IV therapy
Blood cultures negative for
14 days
4-6 weeks IV therapy
(see Endocarditis p. 65)
,iviÀiVi\
Ê-ViÌwVÊ-Ì>ÌiiÌÊÊ >À`Û>ÃVÕ>ÀÊ«>Ì>LiÊ iVÌÀVÊ iÛViÊviVÌÃ\Ê ÀVÕ>ÌÊ
Óä£äÆÊ£Ó£\{xnqÇÇ°
72
TREATMENT
UÊÊANTIBIOTICS SHOULD BE STARTED AS SOON AS THE
POSSIBILITY OF BACTERIAL MENINGITIS BECOMES EVIDENT,
IDEALLY WITHIN 30 MINUTES.
UÊDO NOT WAIT FOR CT SCAN OR LP RESULTS. IF LP MUST BE
DELAYED, GET BLOOD CULTURES AND START THERAPY.
UÊÊ`ÕÃÌÊÌ iÀ>«ÞÊViÊ«>Ì }iÊ>`ÊÃÕÃVi«ÌLÌiÃÊ>ÀiÊÜ°
UÊÊ-iÊ>`ÛV>ÌiÊ«iVÊ`iÃiÃÌâ>ÌÊvÀÊ«>Ì }iëiVwVÊÌ iÀ>«ÞÊ
in patients with severe allergies (p. 137).
UÊÊÌLÌVÊ`ÃiÃÊ>ÀiÊ } iÀÊvÀÊ -ÊviVÌÃÊ­«°ÊÇÇ®°
UÊÊviVÌÕÃÊ Ãi>ÃiÃÊVÃÕÌ>ÌÊÃÊ>`ÛÃi`ÊvÀÊ>Ê -ÊviVÌÃ]Ê
particularly those in which the preferred antibiotic cannot be used or in
which the organism is resistant to usual therapy.
Empiric therapy
Host
Pathogens
Preferred Abx
Immunocompetent*
>}iÊÊxä
Immunocompetent*
age > 50
S. pneumo, N.
mening, H. influenzae
S. pneumo, Listeria,
H. influenzae,
N. mening, Group B
streptococci
S. pneumo, N.
mening, H. influenzae,
Listeria,
(Gram-negatives)
S. pneumo (if CSF
leak), H. influenzae,
Staphylococci,
Gram-negatives
S. aureus, coagulasenegative staphylococci,
Gram-negatives (rare)
Vancomycin PLUS
Ceftriaxone
Vancomycin PLUS
Ceftriaxone PLUS
Ampicillin
Alternative for
serious PCN
allergy (ID consult
recommended)
Moxifloxacin‡ PLUS
Vancomycin
Moxifloxacin‡ PLUS
Vancomycin PLUS
/*É-8
Vancomycin PLUS
Cefepime PLUS
Ampicillin
Vancomycin PLUS
/*É-8ÊPLUS
Ciprofloxacin
Vancomycin PLUS
Cefepime
Vancomycin PLUS
Ciprofloxacin
Vancomycin PLUS
Cefepime
Vancomycin PLUS
Ciprofloxacin
Immunocompromised†
Post neurosurgery or
penetrating head
trauma
Infected shunt
† Immunocompromised is defined as solid organ transplant, BMT in the past year, leukemia
undergoing treatment, or neutropenia
‡ Allergy consult for beta-lactam desensitization
* Use of Dexamethasone
UÊÊ``ÌÊvÊ`iÝ>iÌ >ÃiÊÃÊÀiVi`i`ÊÊ>Ê>`ÕÌÊ«>ÌiÌÃÊÜÌ Ê
suspected pneumococcal meningitis (note that this will be most adult
patients).
UÊÊ Ãi\Êä°£xÊ}É}Ê6Ê+ÈÊvÀÊÓq{Ê`>ÞÃ
UÊÊ/ iÊwÀÃÌÊ`ÃiÊÕÃÌÊLiÊ>`ÃÌiÀi`Ê£äqÓäÊÕÌiÃÊLivÀiÊÀÊ
concomitant with the first dose of antibiotics.
73
6.9 Central nervous system infections
Meningitis – Empiric treatment
6.9 Central nervous system infections
UÊÊ`ÃÌÀ>ÌÊvÊ>ÌLÌVÃÊÃ Õ`ÊÌÊLiÊ`i>Þi`ÊÌÊ}ÛiÊ
dexamethasone.
UÊÊ iÝ>iÌ >ÃiÊÃ Õ`ÊÌÊLiÊ}ÛiÊÌÊ«>ÌiÌÃÊÜ Ê >ÛiÊ>Ài>`ÞÊ
started antibiotics.
UÊÊ ÌÕiÊ`iÝ>iÌ >ÃiÊÞÊvÊÌ iÊ -ÊÀ>ÊÃÌ>ÊÃ ÜÃÊÀ>
positive diplococci or if blood or CSF grows S. pneumoniae
Pathogen-specific therapy (ID consult recommended)
Pathogens
Preferred
S. pneumo PCN MIC ≤ 0.06
μg/ml AND/OR Ceftriaxone
MIC 0.5 μg/ml
S. pneumo PCN MIC ä°£q£Ê
μg/ml AND Ceftriaxone
MIC 1 μg/ml (ID consult
recommended)
S. pneumo PCN MIC 1
μg/ml AND Ceftriaxone
MIC ≥1 μg/ml (ID consult
recommended)
N. meningitidis PCN
susceptible (MIC 0.1)
H. flu
Non -lactamase producer
H. flu
-lactamase producer
Listeria
P. aeruginosa
Penicillin OR Ceftriaxone
E. coli
K. pneumoniae
Enterobacter spp.
S. aureusq--
-°Ê>ÕÀiÕÃq,-Ê
Coagulase-negative
staphylococci if Oxacillin MIC
≤ 0.25
Coagulase-negative
staphylococci Oxacillin MIC
0.25
Enterococcus
Candida species
Cryptococcus
Ceftriaxone
Alternative for serious
PCN allergy (Consult
allergy for PCN skin
testing ± desensitization)
Vancomycin OR
Moxifloxacin OR Linezolid
Ceftriaxone
Moxifloxacin OR Linezolid
Ceftriaxone PLUS
Vancomycin PLUS Rifampin
Moxifloxacin OR Linezolid
Penicillin OR Ceftriaxone³
Consult ID
Ampicillin OR Ceftriaxone
Ciprofloxacin*
Ceftriaxone
Ciprofloxacin*
Ampicillin ±
Cefepime OR Meropenem
Gentamicin‡
Meropenem
Oxacillin
Vancomycin
Oxacillin
/*É-8Ê
Ciprofloxacin PLUS
Aztreonam
Aztreonam OR Ciprofloxacin
",Ê/*É-8
/*É-8ÊÀÊ «ÀyÝ>V
Vancomycin
Vancomycin
Vancomycin
Ampicillin PLUS Gentamicin‡
Amphotericin B
Amphotericin B PLUS
Flucytosine
Vancomycin PLUS Gentamicin‡
* Consider beta-lactam desensitization
³ÊÕÃÌÊ}ÛiÊ «ÀyÝ>VÊxääÊ}ÊViÊÌÊiÀ>`V>ÌiÊV>ÀÀiÀÊÃÌ>ÌiÊvÊ*
‡ Administer aminoglycosides systemically, not intrathecally
74
ÊÕÃi`Ê>ÃÊÌÀi>ÌiÌ
6.9 Central nervous system infections
TREATMENT NOTES
Indications for head CT prior to LP
UÊÃÌÀÞÊvÊ -Ê`Ãi>ÃiÃÊ­>ÃÃÊiÃ]Ê 6®
UÊ iÜÃiÌÊÃiâÕÀiÊ­ 1 week)
UÊ*>«i`i>
UÊÌiÀi`ÊVÃVÕÃiÃÃ
UÊV>ÊiÕÀ}VÊ`iwVÌ
Duration
UÊÊ-/"*ÊÌÀi>ÌiÌÊvÊ*ÊVÕÌÕÀiÊLÌ>i`Ê«ÀÀÊÌÊ>ÌLÌVÊÌ iÀ>«ÞÊÃÊ
negative at 48 hours OR no PMNs on cell count
UÊS. pneumoniae\Ê£äq£{Ê`>ÞÃ
UÊN. meningitidis\ÊÇÊ`>ÞÃ
UÊListeria\ÊÓ£Ê`>ÞÃ
UÊH. influenzae\ÊÇÊ`>ÞÃ
UÊÀ>i}>ÌÛiÊL>V\ÊÓ£Ê`>ÞÃ
Adjunctive therapy
UÊÊ Ã`iÀÊÌÀ>VÀ>>Ê«ÀiÃÃÕÀiÊÌÀ}ÊÊ«>ÌiÌÃÊÜÌ Ê«>Ài`Ê
mental status.
Encephalitis
UÊÊiÀ«iÃÊÛÀÕÃiÃÊ­-6]Ê6<6®ÊÀi>ÊÌ iÊ«Ài`>ÌÊV>ÕÃiÃÊvÊÌÀi>Ì>LiÊ
encephalitis.
UÊ -Ê* ,ÃÊ>ÀiÊÀ>«`Ê`>}ÃÌVÊÌiÃÌÃÊ>`Ê>««i>ÀʵÕÌiÊÃiÃÌÛiÊ>`Ê
specific.
UÊ>ÛiÊÜÊÌ Àià `ÊÌÊÌÀi>ÌÊvÊÃÕëiVÌi`Ê>ÃÊÕÌÀi>Ìi`ÊÀÌ>ÌÞÊ
iÝVii`ÃÊÇ䯰
UÊ/Ài>ÌiÌ\ÊVÞVÛÀÊ£äÊ}É}Ê6Ê+nÊvÀÊ£{qÓ£Ê`>ÞÃ
75
6.9 Central nervous system infections
Brain abscess
UÊÊ «ÀVÊÌÀi>ÌiÌÊÃÊ}Õ`i`ÊLÞÊÃÕëiVÌi`ÊÃÕÀViÊ>`ÊÕ`iÀÞ}Ê
condition. While therapy should be adjusted based on culture results,
anaerobic coverage should ALWAYS continue even if none are grown.
Source/ Condition
Pathogens
Preferred
Unknown
S. aureus,
Streptococci, Gramnegatives, Anaerobes
Streptococci (incl.
S. pneumoniae),
Anaerobes
Gram-negatives,
Streptococci
Anaerobes
Staphylococci, Gram
negatives
Streptococci (esp.
S. viridans)
Vancomycin PLUS
Ceftriaxone PLUS
Metronidazole
Q*iVÊ",Ê
ivÌÀ>ÝiRÊ*1-Ê
Metronidazole
Cefepime PLUS
Metronidazole
Sinusitis
Chronic otitis
Post neurosurgery
Cyanotic heart
disease
Vancomycin PLUS
Cefepime
Penicillin OR
Ceftriaxone
Alternative for
serious PCN allergy
(ID consult
recommended)
Vancomycin PLUS
Ciprofloxacin PLUS
Metronidazole
Vancomycin PLUS
Metronidazole
Aztreonam PLUS
Metronidazole PLUS
Vancomycin
Vancomycin PLUS
Ciprofloxacin
Vancomycin
,iviÀiViÃ\
-ÊÕ`iiÃÊvÀÊ >VÌiÀ>Êi}ÌÃ\Ê ÊviVÌÊ ÃÊÓää{ÆÎ\£ÓÈÇ°
iÝ>iÌ >ÃiÊÊ>`ÕÌÃÊÜÌ ÊL>VÌiÀ>Êi}ÌÃ\Ê Ê }ÊÊi`ÊÓääÓÆÎ{Ç\£x{°
CNS shunt infection
Diagnosis
UÊÊ ÕÌÕÀiÊvÊViÀiLÀë>ÊyÕ`ÊÀi>ÃÊÌ iÊ>ÃÌ>ÞÊvÊ`>}ÃðÊ
Clinical symptoms may be mild and/or non-specific, and CSF
chemistries and leukocyte counts may be normal.
Empiric Therapy
UÊÊ6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊPLUS Cefepime 2 g IV Q8H
OR
UÊÊ* ÊiÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊPLUS
Ciprofloxacin 400 mg IV Q8H
TREATMENT NOTES
UÊID consult recommended for assistance with timing of shunt
replacement and length of antibiotic therapy.
UÊÊ,iÛ>ÊvÊ>ÊV«iÌÃÊvÊÌ iÊviVÌi`ÊÃ ÕÌÊÜÌ ÊiÝÌiÀ>Ê
ventricular drainage or intermittent ventricular taps in combination
with the appropriate intravenous antibiotic therapy leads to the highest
effective cure rates. Success rates are substantially lower when the
infected shunt components are not removed.
76
,iviÀiViÃ\
-ÊÕ`iiÃÊvÀÊÌ iÊ>>}iiÌÊvÊ >VÌiÀ>Êi}ÌÃ\Ê ÊviVÌÊ ÃÊ
Óää{ÆÎ\£ÓÈÇ°Ê
/ iÀ>«ÞÊÊViÀiLÀë>ÊyÕ`Êà ÕÌÊviVÌ°Ê iÕÀÃÕÀ}iÀÞÊ£näÆÇ\{x°
Antimicrobial doses for CNS infections – normal
renal function
Antibiotics
UÊÊ}ÞVÃ`iÃ\ÊÃiiÊ«°Ê£{x
UÊÊ«V\ÊÓÊ}Ê6Ê+{Ê
UÊÊâÌÀi>\ÊÓÊ}Ê6Ê+È
UÊÊ ivÌÀ>Ýi\ÊÓÊ}Ê6Ê+£Ó
UÊÊ ivi«i\ÊÓÊ}Ê6Ê+n
UÊÊ «ÀyÝ>V\Ê{ääÊ}Ê6Ê+nÊ­L>Ãi`ÊÊÌi`Ê`>Ì>®
UÊÊÝyÝ>V\Ê{ääÊ}Ê6Ê+Ó{
UÊÊiÀ«ii\ÊÓÊ}Ê6Ê+n
UÊÊiÌÀ`>âi\ÊxääÊ}Ê6Ê+È
UÊÊ"Ý>V\ÊÓÊ}Ê6Ê+{
UÊÊ*iV\Ê{ÊÊÕÌÃÊ6Ê+{Ê­Ó{ÊÊÕÌÃÊ«iÀÊ`>Þ®
UÊÊ,v>«\ÊÈääÊ}Ê6Ê+£ÓqÓ{
UÊÊ/*É-8\ÊxÊ}É}Ê­/*ÊV«iÌ®Ê6Ê+È
UÊÊ6>VÞV\Ê>`ÊÜÌ ÊÓxqÎxÊ}É}]ÊÌ iÊ£xqÓäÊ}É}Ê+nq£ÓÊ
(minimum 1 g Q12H)
UÊÊ6>VÞVÊÃ Õ`ÊLiÊ>`ÃÌiÀi`ÊÌÊ>Ì>ÊÃiÀÕÊÌÀÕ} Ê
concentrations close to 20 mcg/mL.
Antifungals
UÊÊ« ÌiÀV\Êä°Çq£Ê}É}Ê6Ê+Ó{
UÊ Ãi®\ÊÎ{Ê}É}Ê6Ê+Ó{ÊvÀÊ ÀÞ«ÌVVV>Êi}ÌÃ
UÊÊ Ãi®\ÊxÊ}É}Ê6Ê+Ó{ÊvÀÊ >``>Êi}ÌÃ
UÊÕV>âi\Ênääq£ÓääÊ}Ê6É*"Ê+Ó{Ê­V>Ê}ÛiÊÊ`Û`i`Ê`Ãiî
UÊÊÕVÞÌÃi\ÊÓxÊ}É}Ê*"Ê+È
Intraventricular antibiotics (ID consult recommended)
UÊÊ>V\ÊÎäÊ}Ê+Ó{Ê­VÌ>ÃÊ«ÀiÃiÀÛ>ÌÛi®
UÊÊiÌ>V\ÊxÊ}Ê+Ó{
UÊÊ/LÀ>ÞV\ÊxÊ}Ê+Ó{
UÊÊ6>VÞV\ÊÓäÊ}Ê+Ó{
77
6.9 Central nervous system infections
UÊÊ/ iÊÀiÊvÊÌÀ>ÛiÌÀVÕ>ÀÊ>ÌLÌVÃÊÃÊVÌÀÛiÀÃ>]Ê>`Ê}iiÀ>ÞÊ
limited to refractory cases or cases in which shunt removal is not
possible. Intraventricular injection should be administered only by
experienced physicians.
6.10 Acute bacterial rhinosinusitis
Acute bacterial rhinosinusitis (ABRS)
NOTE: Sinusitis in immunocompromised hosts can be caused by fungi
>`ÊÌ iÀÊiÃÃVÊ«>Ì }iÃÆÊVÃÕÌ>ÌÊÜÌ Ê Ê>`Ê /ÊÃÊ
recommended to guide management and therapy.
ÃÌÊÀ ÃÕÃÌÃÊ`iÃÊÌÊÀiµÕÀiÊ>ÌLÌVÊÌÀi>ÌiÌÆÊÌÀi>ÌiÌÊ
à Õ`ÊLiÊVÃ`iÀi`ÊÊÌ iÊvÜ}ÊÃVi>ÀÃ\
UÊ*iÀÃÃÌiÌÊÃÞ«ÌÃÊvÊ>VÕÌiÊÀ ÃÕÃÌÕÃÊ≥ 10 days without
improvement
UÊiÛiÀÊ≥39°C and purulent nasal discharge or facial pain lasting >3-4
days from the beginning of illness
UÊ iÜÊÃiÌÊvÊviÛiÀ]Ê i>`>V iÊÀÊVÀi>ÃiÊÊ>Ã>Ê`ÃV >À}iÊvÜ}Ê
viral URI that lasted 5-6 days and was initially improving
EMPIRIC TREATMENT
Oral regimens
UÊÝVÉV>ÛÕ>>ÌiÊnÇxÊ}Ê*"Ê+£Ó
OR
UÊÝVÉV>ÛÕ>>ÌiÊ8,ÊÓÊ}Ê*"Ê+£ÓÊÊvÀÊ«>ÌiÌÃÊÜÌ ÊÃiÛiÀiÊ
infection (e.g. systemic toxicity with fever of 39°C), antibiotic use in
previous 30 days, immunocompromised
OR
UÊ ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê iv«`ÝiÊÓääÊ}Ê*"Ê+£Ó
OR
UÊ-iÛiÀiÊ* Ê>iÀ}Þ\ÊÝyÝ>VÊ{ääÊ}Ê*"Ê`>ÞÊÊ
Parenteral regimens
UÊ«VÉÃÕL>VÌ>Ê£°xÊ}Ê6Ê+È
OR
UÊ ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê ivÌÀ>ÝiÊ£Ê}Ê6Ê+Ó{
OR
UÊ-iÛiÀiÊ* Ê>iÀ}Þ\ÊÝyÝ>VÊ{ääÊ}Ê6Ê+Ó{ÊÊ
Duration
UÊxÇÊ`>ÞÃÊ
TREATMENT NOTES
Microbiology
UÊ*Ài`>ÌÞÊS. pneumoniae, H. influenzae, M. catarrhalis
UÊÀ>i}>ÌÛiÊiÌiÀVÊL>VÊ>ÀiÊÀ>Ài
Management
UÊ ,-ÊÃÊÀ>ÀiÞÊ«ÀiÃiÌÊ«ÀÀÊÌÊÇq£äÊ`>ÞÃÊvÊÃÞ«ÌÃÆÊÌÞ«V>Ê
inciting etiologies of acute sinusitis include allergies and viral URI
78
,iviÀiVi\Ê
-Ê}Õ`iiÃÊvÀÊ ,-°Ê ÊviVÌÊ ÃÊÓä£ÓÆÊx{­n®\iÇÓi££Ó°Ê
79
6.10 Acute bacterial rhinosinusitis
UÊ ÕÌÕÀiÃÊLÞÊ`ÀiVÌÊÃÕÃÊ>ëÀ>ÌÊÀÊi`ÃV«V>ÞÊ}Õ`i`ÊVÕÌÕÀiÊvÊ
the middle meatus should only be obtained in patients who fail empiric
antibiotic therapy. Nasopharyngeal swab is NOT recommended for
obtaining culture data.
UÊ wÀ>ÌÊvÊ`>}ÃÃÊÜÌ Ê>}}ÊÃÊÌÊÀiVi`i`ÊvÀÊ
uncomplicated ABRS. Consider CT in those with severe disease with
possible extension to the orbit or intracranial space.
UÊÌÀ>>Ã>ÊÃ>iÊÀÀ}>ÌÊ­« ÞÃ}VÊÀÊ Þ«iÀÌV®Ê>`ÊÌÀ>>Ã>Ê
corticosteroids are recommended as an adjuncts to antibiotic therapy
and can also provide symptomatic relief in patients in whom antibiotic
are not indicated
UÊ>VÀ`iÃÊ­ >ÀÌ ÀÞV]ÊâÌ ÀÞV®Ê>ÀiÊÌÊÀiVi`i`ÊvÀÊ
initial empiric therapy due to high rates of resistance of S. pneumoniae
­xx¯Ê>ÌÊ®
UÊ iëÌiÊ -Ê}Õ`iiÃÊÃÕ««ÀÌ}ÊÕÃiÊvÊ ÝÞVÞViÊ>ÃÊ>Ê
alternative agent for ABRS, Doxycycline is NOT recommended for
initial empiric therapy at JHH due to high rates of resistance of S.
pneumoniae ­Óǯ®Ê>` H. influenzae ­Îx¯®
UÊ,ÕÌiÊVÛiÀ>}iÊvÀÊ,-ÊÊÌ>Êi«ÀVÊÌ iÀ>«ÞÊvÀÊ ,-ÊÊÌÊ
recommended
6.11 Orbital cellulitis
Orbital cellulitis
Preseptal cellulitisÊ­ä¯ÊvÊV>Ãiî
UÊÛÛiÃÊÌÃÃÕiÃÊ>ÌiÀÀÊÌÊÌ iÊÀLÌ>ÊÃi«ÌÕÊ
UÊ*ÀiÃiÌÃÊÜÌ ÊviÛiÀ]ÊiÞi`ÊiÀÞÌ i>Ê>`ÊÃvÌÊÌÃÃÕiÊÃÜi}ÊLÕÌÊÊ
orbital congestion
Postseptal cellultis
UÊ-}ÃÊvÊ«iÀÀLÌ>ÊViÕÌÃÊ>ÃÊÜiÊ>ÃÊÌ>ÌÊvÊVÕ>ÀÊÛiiÌÃ]Ê
pain with ocular movement, and/or proptosis
UÊ-iÛiÀiÊviVÌÊV>Ê>ÃÊÛÛiÊÛÃÕ>ÊÃÃ]ÊÃÕL«iÀÃÌi>Ê>LÃViÃÃ]Ê
globe displacement, abscess formation
UÊ"vÌiÊ>ÃÃV>Ìi`ÊÜÌ ÊÃÕÃÌÃÊ
UÊ >ÊLiÊ>ÃÃV>Ìi`ÊÜÌ ÊV>ÛiÀÕÃÊÃÕÃÊÌ ÀLÃÃ
EMPIRIC TREATMENT
UÊ«VÉÃÕL>VÌ>ÊÎÊ}Ê6Ê+È
OR
UÊ ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê ivÌÀ>ÝiÊÓÊ}Ê6Ê`>Þ
OR
UÊ-iÛiÀiÊ* Ê>iÀ}Þ\ÊÝyÝ>VÊ{ääÊ}Ê6Ê`>Þ
Add Vancomycin (see dosing section, p. 150) in patients with history
of MRSA colonization or infection, evidence of abscess or bone
involvement, orbital trauma, recent ophthalmic surgery or severe
infection
Oral step down therapy (for patients without culture data to guide
therapy and without evidence of bony involvement or cavernous sinus
thrombosis)
UÊÝVÉV>ÛÕ>>ÌiÊnÇxÊ}Ê*"Ê+£Ó
OR
UÊ ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê iv«`ÝiÊ{ääÊ}Ê*"Ê+£Ó
OR
UÊ-iÛiÀiÊ* Ê>iÀ}Þ\ÊÝyÝ>VÊ{ääÊ}Ê*"Ê`>Þ
Duration
UÊÇÊ`>ÞÃÊÕ«ÊÌÊÈÊÜiiÃÊvÊiÛ`iViÊvÊLÞÊÛÛiiÌ
TREATMENT NOTES
Microbiology
UÊS. aureus, beta-hemolytic streptococci, S. pneumoniae, H. influenza,
M. catarrhalis (cultures are infrequently positive)
Management
UÊ>}}ÊÃÊÀiVi`i`ÊÊ«ÃÌÃi«Ì>ÊViÕÌÃÊ­ /ÊÀÊ,®
UÊ ÃÕÌ>ÌÊÜÌ Ê ]Ê /]Ê>`Ê« Ì >}ÞÊÀiVi`i`
80
81
6.11 Orbital cellulitis
UÊ*ÃÌÃi«Ì>ÊViÕÌÃÊÊÕV«ÀÃi`Ê ÃÌÃÊV>ÊLiÊV>ÕÃiÊ
LÞÊvÕ}Ê>`Ê`ÃÆÊi«ÀVÊ>ÌvÕ}>ÊÌ iÀ>«ÞÊÃÊÀiVi`i`ÊÊ
consultation with ID
UÊ*ÃÌÃi«Ì>ÊViÕÌÃÊÜÌ Ê>LÃViÃÃÊvÀ>ÌÊÃ Õ`Ê«À«ÌÊi`>ÌiÊ
surgical intervention
UÊ,iëÃiÊÌÊ>««À«À>ÌiÊ>ÌLÌVÊÌ iÀ>«ÞÊà Õ`ÊVVÕÀÊÊÓ{ÊqÊ{nÊ
hours
UÊ*ÀÊÀiëÃiÊÌÊ>ÌLÌVÃ]ÊÜÀÃi}ÊÛÃÕ>Ê>VÕÌÞÊÀÊ«Õ«>ÀÞÊ
changes and/or evidence of an abscess are indications for surgery
6.12 Pulmonary infections
COPD exacerbations
EMPIRIC TREATMENT
UÊÊÊDoxycycline 100 mg PO BID for 5 days
OR
UÊÊâÌ ÀÞVÊxääÊ}Ê*"É6Ê+Ó{ÊvÀÊÎÊ`>ÞÃ
OR
UÊÊÝVÉV>ÛÕ>>ÌiÊnÇxÊ}Ê*"Ê ÊvÀÊxÊ`>ÞÃ
OR
UÊÊ iv«`ÝiÊÓääÊ}Ê*"Ê ÊvÀÊxÊ`>ÞÃ
OR
UÊ iv`ÀÊÎääÊ}Ê*"Ê ÊvÀÊxÊ`>ÞÃ
TREATMENT NOTES
Microbiology
UÊÊ*Ài`>ÌÞÊH. influenzae, M. catarrhalis, S. pneumoniae
UÊÊPseudomonas, Enterobacteriaceae are less common and seen in
patients with severe COPD and extensive antibiotic exposure.
Management
UÊÊ «ÀVÊÕÃiÊvÊyÕÀµÕiÃÊÃÊ`ÃVÕÀ>}i`Ê>`Êà Õ`ÊÞÊ
be considered if past or present microbiologic evidence indicates
infection with a pathogen(s) that is resistant to standard therapy (e.g.
Pseudomonas, Enterobacteriaceae).
UÊÊ6Ê>ÌLÌVÃÊà Õ`ÊÞÊLiÊÕÃi`ÊvÊÌ iÊ«>ÌiÌÊV>ÌÊÌiÀ>ÌiÊ*"Ê
antibiotics.
UÊÊÌLÌVÃÊ>ÀiÊÌÊ`V>Ìi`ÊvÀÊ>ÃÌ >Êy>ÀiÃÊÊÌ iÊ>LÃiViÊvÊ
pneumonia.
Prophylactic antibiotics for the prevention of COPD exacerbations
UÊ*À« Þ>VÌVÊ>ÌLÌVÃÊ >ÛiÊLiiÊÃ ÜÊÌÊÀi`ÕViÊÀ>ÌiÃÊvÊ
exacerbations and improve reported quality of life but not to decrease
all-cause or respiratory-associated mortality
UÊ*À}i`ÊâÌ ÀÞVÊÕÃiÊ >ÃÊLiiÊ>ÃÃV>Ìi`ÊÜÌ Ê i>À}ÊÃÃÊ
>`Ê+/Ê«À}>ÌÆÊ«>ÌiÌÃÊÜÌ ÊL>ÃiiÊ+/«À}>ÌÊÜiÀiÊÌÊ
included in clinical trials
UÊ/ iÊ`iVÃÊÌÊÌ>ÌiÊ«À« Þ>VÌVÊ>ÌLÌVÃÊà Õ`ÊLiÊ>`iÊÊ>Ê
case-by-case basis and should take in to account patient preferences,
financial constraints, risk factors for adverse events and input from the
patient’s pulmonologist
UÊ,iVi`i`ÊÀi}i\ÊâÌ ÀÞVÊÓxäÊ}Ê*"Ê`>Þ
UÊ >ÃiiÊ>Õ`iÌÀÞÊ>`Ê ÊÃÊÀiVi`i`
,iviÀiViÃ\
iÀV>Ê i}iÊvÊ* ÞÃV>ÃÊ*ÃÌÊ*>«iÀ\ÊÊÌiÀÊi`ÊÓää£ÆÊ£Î{\Èää°
ÕÀ>ÌÊvÊÌ iÀ>«Þ\Ê/ À>ÝÊÓäänÆÊÈέx®\{£xqÓÓ°
âÌ ÀÞVÊvÀÊ«ÀiÛiÌ\Ê °Ê }°ÊÊi`ÊÓ䣣ÆÊÎÈx\ÊÈnÆÊ V À>iÊ >Ì>L>ÃiÊ-ÞÃÌÊ
Rev 2013 Nov 28.
82
NOTE: If patient is coming from a nursing home or long-term care
facility, see Healthcare-acquired pneumonia, p. 87.
EMPIRIC TREATMENT
Patient NOT in the ICU
UÊ«VÉÃÕL>VÌ>Ê£°xÊ}Ê6Ê+ÈÊPLUS Azithromycin 500 mg IV/PO
once daily
OR
UÊÊ ivÌÀ>ÝiÊ£Ê}Ê6Ê+Ó{ÊPLUS Azithromycin 500 mg IV/PO once daily
OR
UÊÝyÝ>VÊ{ääÊ}Ê6É*"Ê+Ó{Ê
In non-critically ill patients, consider switch to oral agents as soon as patient
is clinically improving and eating (see next page for oral options and doses).
Patient in the ICU
Not at risk for infection with Pseudomonas (see risks below)
UÊÊ ivÌÀ>ÝiÊ£Ê}Ê6Ê+Ó{ÊPLUS Azithromycin 500 mg IV Q24H
OR
UÊÊ* Ê>iÀ}Þ\ÊÝyÝ>VÊ{ääÊ}Ê6Ê+Ó{Ê
At risk for infection with Pseudomonas (see risks below)
UÊÊ ivi«iÊ£ÓÊ}Ê6Ê+nÊPLUS Azithromycin 500 mg IV Q24H
OR
UÊÊ*«iÀ>VÉÌ>âL>VÌ>Ê{°xÊ}Ê6Ê+ÈÊPLUS Azithromycin 500 mg IV Q24H
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\ÊÝyÝ>VÊ{ääÊ}Ê6Ê+Ó{ÊPLUS Aztreonam
2 g IV Q8H
UÊÊ-«ÕÌÕÊ}À>ÊÃÌ>Ê>ÞÊ i«Ê`iÌiÀiÊvÊPseudomonas is present.
UÊÊNarrow coverage if Pseudomonas is NOT present on culture at 48 hours.
Risks for PseudomonasÊ>`ÊÌ iÀÊÀiÃÃÌ>ÌÊÀ>i}>ÌÛiÊÀ}>ÃÃ\
LÀV iVÌ>ÃÃÆÊLÀ>`ëiVÌÀÕÊ>ÌLÌVÃÊvÀÊÊÇÊ`>ÞÃÊÊÌ iÊ«>ÃÌÊ
Ì ÆÊ«À}i`Ê Ã«Ì>â>ÌÊÊÇÊ`>ÞÃÆÊ`iLÌ>Ìi`ÊÕÀÃ}Ê iÊ
ÀiÃ`iÌÆÊÀiViÌÊiV >V>ÊÛiÌ>ÌÊÊ{nÊÆÊÕV«ÀÃi`Ê
due to solid organ transplant, hematologic malignancy, BMT, active
chemotherapy, prednisone > 20 mg daily for > 3 weeks.
DIAGNOSIS
UÊÊÕV«iÌiÌÊ«>ÌiÌÃÊ1-/Ê >ÛiÊ>ÊV iÃÌÊ8À>ÞÊwÌÀ>ÌiÊÌÊiiÌÊ
diagnostic criteria for pneumonia.
UÊÊ-«ÕÌÕÊ>`ÊL`ÊVÕÌÕÀiÃÊÃ Õ`ÊLiÊÃiÌÊÊ>Ê«>ÌiÌÃÊ>`ÌÌi`ÊÌÊ
the hospital BEFORE antibiotics are given.
UÊÊS. pneumoniae urine antigen should be obtained in all patients with CAP.
ÌÊ >ÃÊëiVwVÌÞÊvÊȯÊ>`Ê«ÃÌÛiÊ«Ài`VÌÛiÊÛ>ÕiÊvÊnn°nÈ°x¯°ÊÌÊ
is particularly useful if antibiotics have already been started or cultures
cannot be obtained.
83
6.12 Pulmonary infections
Community-acquired pneumonia (CAP) in
hospitalized patients
6.12 Pulmonary infections
UÊÊ/ iÊi}i>ÊÕÀiÊ>Ì}iÊÃÊÌ iÊÌiÃÌÊvÊV ViÊvÀÊ`>}Ã}Ê
legionella infection. This test detects only L. pneumophila serogroup
£]ÊÜ V ÊÃÊÀiëÃLiÊvÀÊÇäqnä¯ÊvÊviVÌð
DURATION
UÊ/ iÀ>«ÞÊV>ÊLiÊÃÌ««i`Ê>vÌiÀÊÌ iÊ«>ÌiÌÊÃ\
Ê UÊviLÀiÊvÀÊ{nqÇÓÊ ÕÀÃ
AND
Ê UÊÊ>ÃÊÊÀiÊÌ >ÊiÊvÊÌ iÊvÜ}ÊÃ}ÃÊ>`ÊÃÞ«ÌÃ\Ê,Ê
100 beats/min, RR 24 breaths/min, BP 90 mmHg, O2 sat
Êä¯]Ê>ÌiÀi`ÊiÌ>ÊÃÌ>ÌÕðÊ
UÊÊ-Õ}}iÃÌi`Ê`ÕÀ>ÌÊvÊÌ iÀ>«ÞÊL>Ãi`ÊÊ«>ÌiÌÊëiVwVÊv>VÌÀÃ\
Ê UÊÊ3–5 days: Patient without immunocompromise or structural lung
disease
Ê UÊÊ7 days: Patients with moderate immunocompromise and/or
structural lung disease
Ê UÊÊ10–14 days: Patients with poor clinical response, who
received initial inappropriate therapy, or who are significantly
immunocompromised
UÊÊ1V«V>Ìi`ÊL>VÌiÀiVÊ«iÕVVV>Ê«iÕ>qÊ«À}i`Ê
course of antibiotic therapy not necessary, treat as pneumonia
UÊÊ Õ} Ê>`ÊV iÃÌÊ8À>ÞÊ>LÀ>ÌiÃÊ>ÞÊÌ>iÊ{qÈÊÜiiÃÊÌÊ«ÀÛi°Ê
There is NO need to extend antibiotics if the patient is doing well
otherwise (e.g. no fever).
Other causes of pneumonia
UÊÊ-ÕëiVÌi`Ê>ëÀ>Ì\ Additional empiric coverage for aspiration is justified
only in classic aspiration syndromes suggested by loss of consciousness
(overdose, seizure) PLUS gingival disease or esophageal motility disorder.
Ceftriaxone, Cefepime, and Moxifloxacin have adequate activity against
most oral anaerobes. For classic aspiration, Clindamycin 600 mg IV Q8H
can be added to regimens not containing Piperacillin/tazobactam.
UÊÊ ÕÌÞ>VµÕÀi`Ê,-\ Necrotizing pneumonia with cavitation in
absence of risk factors for aspiration listed above is concerning for
CA-MRSA pneumonia, particularly if associated with a preceding or
concomitant influenza-like illness. In these cases, Linezolid 600 mg IV/PO
Q12H can be added while awaiting culture data. Infectious Diseases
consult is strongly recommended. Use of Linezolid monotherapy for
MRSA bacteremia, even if associated with a pulmonary source, is not
recommended. In the absence of necrotizing pneumonia with cavitation,
empiric coverage for CA-MRSA can be deferred until sputum and blood
culture results return given their high diagnostic yield for CA-MRSA.
UÊÊ,iëÀ>ÌÀÞÊÛÀÕÃiÃ\ Respiratory viruses can cause primary viral
pneumonia as well as lead to bacterial superinfection. Strongly consider
testing all patients with CAP during respiratory virus season (see p. 93).
,iviÀiViÃ\
-É/-Ê ÃiÃÕÃÊÕ`iiÃÊvÀÊ *\Ê ÊviVÌÊ ÃÊÓääÇÆ{{\-ÓÇ°
S. pneumo >Ì}i\ÊÀV ÊÌiÀÊi`ÊÓ䣣ƣǣ­Ó®\£ÈÈqÇÓ
ÎÊ`>ÞÃÊvÊÌ iÀ>«ÞÊvÀÊ *\Ê ÊÓääÈÆÎÎÓ\£Îxx°
84
85
Ceftriaxone 1 g IV Q24
OR
Cefpodoxime 200 mg PO BID
OR
Cefdinir 300 mg PO BID
«VÊ£Ê}Ê6Ê+ÈÊ
OR
Amoxicillin 500 mg PO TID
Ê
S. pneumoniae PCN resistant,
cephalosporin susceptible
Ê
H. influenzae LiÌ>>VÌ>>ÃiÊÊ
producing (Ampicillin susceptible)
Penicillin G 1 million units IV Q6H
OR
Amoxicillin 1 g PO TID
S. pneumoniae PCN intermediate
or urine antigen positive
Amoxicillin 500 mg PO TID
Ê
Ê
Penicillin G 1 million units IV Q6H
OR
Ê
Ê
Preferred therapy
S. pneumoniae PCN susceptible
Pathogen-specific and step-down therapy
Organism
PCN allergy
âÌ ÀÞVIQxääÊ}Ê*"Ê`>ÞÊ8ÊÎÊ`>ÞÃÊ",ÊÊ
xääÊ}ÊVi]ÊÌ iÊÓxäÊ}Ê*"Ê`>ÞÊ8Ê{Ê`>ÞÃR
ORÊ
iv«`ÝiÊÓääÊ}Ê*"Ê ÊÊÊ
OR
Cefdinir 300 mg PO BID
OR
Doxycycline† 100 mg PO BID
OR
Moxifloxacin 400 mg IV/PO daily
(if resistant to other options)
Moxifloxacin 400 mg IV/PO Q24H
Same as above
Non-severe reaction:ÊÊ
Cefpodoxime 200 mg PO BID
OR
Cefdinir 300 mg PO BID
Severe reaction:
âÌ ÀÞVIQxääÊ}Ê*"Ê`>ÞÊÊ8ÊÎÊ`>ÞÃÊÊ
",ÊxääÊ}ÊVi]ÊÌ iÊÓxäÊ}Ê*"Ê`>ÞÊ8Ê{Ê`>ÞÃRÊ
OR
Moxifloxacin 400 mg IV/PO daily
(if Erythromycin resistant)
Notes
6.12 Pulmonary infections
Çx¯ÊvÊH. influenzae isolates at JHH
(excluding oncology) are susceptible to
«V]Ê£ää¯ÊÌÊ ivÌÀ>Ýi]ÊÈx¯ÊÌÊ
/iÌÀ>VÞVi]Ê>`Ê£ää¯ÊÌÊÝyÝ>VÊ
None of the S. pneumoniae isolates at
(excluding oncology) are resistant JHH
to PCN
£¯ÊvÊS. pneumoniae isolates at JHH
(excluding oncology) are susceptible and
¯Ê>ÀiÊÌiÀi`>ÌiÊÌÊ* ]Ê{x¯Ê>Ài
susceptible to Erythromycin (Erythromycin
susceptibilities predict Azithromycin
ÃÕÃVi«ÌLÌiÃÊvÀÊS. pneumoniae), and
£ää¯Ê>ÀiÊÃÕÃVi«ÌLiÊÌÊÝyÝ>V
86
PCN allergy
Azithromycin 500 mg IV/PO Q24H x 7-10 days
OR
ÝyÝ>VÊ{ääÊ}Ê6É*"Ê+Ó{Ê8Ê£ä£{Ê`>ÞÃ
âÌ ÀÞVIQxääÊ}Ê*"Ê`>ÞÊ8ÊÎÊ`>ÞÃÊ",Ê
xääÊ}ÊVi]ÊÌ iÊÓxäÊ}Ê*"Ê`>ÞÊ8Ê{Ê`>ÞÃR
OR
Cefpodoxime 200 mg PO BID
OR
Cefdinir 300 mg PO BID
OR
Doxycycline† 100 mg PO BID
OR
Moxifloxacin 400 mg IV/PO Q24H
(if resistant to other options)
iv«`ÝiÊÓääÊ}Ê*"Ê ÊÊ
ÝyÝ>VÊ{ääÊ}Ê6É*"Ê+Ó{Ê
OR
Cefdinir 300 mg PO BID
OR
ÝVÉV>ÛÕ>>ÌiÊ8,ÊÓÊ}Ê*"Ê Ê
Ê
Ê
Ê
Ìi\Ê1iÃÃÊÃÌÀ}ÊÃÕëVÊvÀÊÊ
Ê
L. pneumophilia, more than 3 days of
Azithromycin for atypical coverage is not
needed due to very long half-life in lung tissue
IvÊ ÀÞÌ ÀÞVÊÃÕÃVi«ÌLiÆÊaÊvÊ/iÌÀ>VÞViÊÃÕÃVi«ÌLi
Ê
Ê
Ê
ÕÌÕÀiÊ>`ÊÕÀiÊ>Ì}iÊi}>ÌÛiÊ
Azithromycin 500 mg IV/PO Q24H
OR
ÝyÝ>VÊ{ääÊ}Ê6É*"Ê+Ó{Ê
L. pneumophilia
Ê
Preferred therapy
«VÉÃÕL>VÌ>Ê£°xÊ}Ê+ÈÊ
ORÊ
Amoxicillin/clavulanate 875 mg PO BID
H. influenzae LiÌ>>VÌ>>ÃiÊÊ
producing (Ampicillin resistant)
Pathogen-specific and step-down therapy
Organism
{x¯ÊvÊS. pneumoniae isolates at JHH
(excluding oncology) are susceptible to
Erythromycin (Erythromycin susceptibilities
predict Azithromycin susceptibilities for
S. pneumoniae®Ê>`ÊÇίÊ>ÀiÊÃÕÃVi«ÌLiÊ
ÌÊ/iÌÀ>VÞViÆÊÌ iÀivÀi]ÊÌ iÃiÊ>}iÌÃ
>ÀiÊÃÕL«Ì>ÊvÀÊi«ÀVÊÃÌi«`Ü
therapy
Notes
6.12 Pulmonary infections
NOTE: If the patient is on antibiotic therapy or has recently been on
antibiotic therapy, choose an agent from a different class.
EMPIRIC TREATMENT
Patient with mild to moderate illness (e.g., not in or transferring to
the ICU/intermediate care unit, no or minimal oxygen requirement, no
hypotension)
UÊ ivÌÀ>ÝiIÊ£Ê}Ê6Ê+Ó{
OR
UÊ-iÛiÀiÊ* Ê>iÀ}Þ\ÊÝyÝ>VÊ{ääÊ}Ê6É*"Ê+Ó{
Patient with severe illness (e.g., in or transferring to the ICU/
intermediate care unit, concern for sepsis, significant oxygen
requirement, multi-lobar consolidation)
UÊ ivi«iIÊÓÊ}Ê6Ê+nʱ Vancomycin† (see dosing section, p. 150)
OR
UÊ*«iÀ>VÉÌ>âL>VÌ>IÊ{°xÊ}Ê6Ê+Èʱ Vancomycin† (see dosing
section, p. 150)
OR
UÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊPLUS
Ciprofloxacin 400 mg IV Q8H ± Gentamicin (see dosing section, p. 146)
*Consider adding Azithromycin 500 mg IV/PO Q24H if the patient is immunosuppressed
or coming from a nursing home or long term care facility to cover Legionella
†Add Vancomycin in patients with a history of MRSA colonization or infection,
necrotizing pneumonia, pneumonia after a respiratory viral illness, ill patients coming
from a nursing home or long term care facility, sepsis)
Patient with history of or risk factors for Pseudomonas and other
resistant Gram-negative organismsÊ­i°}°]ÊLÀV iVÌ>ÃÃÆÊLÀ>`ëiVÌÀÕÊ
>ÌLÌVÃÊvÀÊÊÇÊ`>ÞÃÊÊÌ iÊ«>ÃÌÊÌ ÆÊ«À}i`Ê Ã«Ì>â>ÌÊÊ
ÇÊ`>ÞÃÆÊ`iLÌ>Ìi`ÊÕÀÃ}Ê iÊÀiÃ`iÌÆÊÀiViÌÊiV >V>ÊÛiÌ>ÌÊ
Ê{nÊ ÕÀÃÆÊÕV«ÀÃi`Ê`ÕiÊÌÊÃ`ÊÀ}>ÊÌÀ>ë>Ì]Ê
hematologic malignancy, BMT, active chemotherapy, prednisone > 20
}Ê`>ÞÊvÀÊÊÎÊÜiiî\ÊÌÀi>ÌÊ>ÃÊÃiÛiÀiÊiÃÃÊÜÌ ÊÌ>À}ÊvÊ>ÌLÌVÊ
based on past culture data
NOTE: Always narrow therapy based on cultures results
Oral step down therapy (if no sputum culture data to guide therapy)
UÊÊ iv«`ÝiÊ{ääÊ}Ê*"Ê Ê­vÊÊ ivÌÀ>Ýi®Ê",ÊÝyÝ>VÊ{ääÊ
mg PO daily
Duration:ÊvÊ«iÕ>ÊVwÀi`ÊxÇÊ`>ÞÃÆÊvÊ«iÕ>Ê`>}ÃÃÊÃÊ
questionable and patient improves, can considered stopping therapy
after 3 days
TREATMENT NOTES
Microbiology
UÊÊ ÌiÀVVVÊ>`ÊV>``>ÊëiViÃÊ>ÀiÊvÌiÊÃ>Ìi`ÊvÀÊÌ iÊëÕÌÕÊ
in hospitalized patients. In general, they should be considered to be
colonizing organisms and should not be treated with antimicrobials.
87
6.12 Pulmonary infections
Healthcare-acquired pneumonia
(NOT ventilator-associated)
6.12 Pulmonary infections
Antimicrobial management of “aspiration events”
UÊ*À« Þ>VÌVÊ>ÌLÌVÃÊ, Ê "/ÊÀiVi`i`ÊvÀÊ«>ÌiÌÃÊÜ Ê>ÀiÊ
at increased risk for aspiration.
UÊi`>ÌiÊÌÀi>ÌiÌÊÃÊ`V>Ìi`ÊvÀÊ«>ÌiÌÃÊÜ Ê >ÛiÊÃ>LÜiÊ
obstructions or are on acid suppression therapy given the increased
risk of gastric colonization.
UÊÌLÌVÊÌÀi>ÌiÌÊvÊ«>ÌiÌÃÊÜ Ê`iÛi«ÊviÛiÀ]ÊiÕVÞÌÃÃÊ>`Ê
infiltrates in the first 48 hours after an aspiration is likely unnecessary
since most aspiration pneumonias are chemical and antibiotic
treatment may only select for more resistant organisms.
UÊ/Ài>ÌiÌÊ-ÊÀiVi`i`ÊvÀÊ«>ÌiÌÃÊÜ Ê >ÛiÊÃÞ«ÌÃÊvÀÊ
more than 48 hours or who are severely ill.
,iviÀiViÃ\
ëÀ>ÌÊ«iÕÌÃÊ>`Ê>ëÀ>ÌÊ«iÕ>\Ê Ê }ÊÊi`ÊÓää£ÆÎ{{­®\ÈÈx°
/-É -ÊÕ`iiÃÊvÀÊ*É6*\Ê, ÊÓääxƣǣ\Înn°
Ventilator-associated pneumonia (VAP)
UÊÊ-«ÕÌÕÊVÕÌÕÀiÃÊÃ Õ`ÊLiÊLÌ>i`Ê«ÀÀÊÌÊÃÌ>ÀÌ}Ê>ÌLÌVÃÊÀÊ
if patient is failing therapy by endotracheal suction or invasive
techniques. ET suction appears just as sensitive but less specific
than invasive methods.
UÊÊEmpiric treatment MUST be narrowed as soon as sputum
culture results are known.
UÊÊvÊÌ iÊ«>ÌiÌÊÃÊÊ>ÌLÌVÊÌ iÀ>«ÞÊÀÊ >ÃÊÀiViÌÞÊLiiÊÊ>ÌLÌVÊ
therapy, choose an agent from a different class.
Optimal treatment can likely be based on severity of illness as
determined by the Clinical Pulmonary Infection Score (CPIS).
Calculating the Clinical Pulmonary Infection Score (CPIS)
Temperature (°C)
Peripheral WBC
0 points
36.5 to 38.4
{]äääÊqÊ££]äää
Tracheal
secretions
Chest X-ray
None
Progression
of infiltrate
from prior
radiographs
Culture of ET
suction
None
Oxygenation
(PaO2/FiO2)
> 240 or ARDS
88
No infiltrate
No growth/light
growth
2 points
1 point
≤ 36.4 or ≥ 39
38.5 to 38.9
Ê{]äääÊÀÊ
> 11,000
> 50% bands: add
1 extra point
Purulent
Non-purulent
Diffuse or patchy
infiltrates
Localized
infiltrate
Progression
(ARDS, CHF
thought unlikely)
Heavy growth
Same bacteria on
gram stain: add 1
extra point
≤ 240 and no
ARDS
If the CPIS is ≤ 6
UÊÊ6*ÊÃÊÕiÞ
UÊÊvÊ6*ÊÃÌÀ}ÞÊÃÕëiVÌi`ÊÃiiÊÌÀi>ÌiÌÊÀiVi`>ÌÃÊLiÜ
UÊÊvÊ *-ÊÀi>ÃÊ≤ 6 after 3 days, antibiotics can be stopped in most
cases
If the CPIS is > 6
Early-onset VAP (occurring within 72 hours of hospitalization and
patient has not been hospitalized or resided in a nursing home, longterm care or rehabilitation facility in the past 3 months)
Etiology: S. pneumoniae, H. influenzea, S. aureus
UÊ ivÌÀ>ÝiÊ£Ê}Ê6Ê+Ó{
OR
UÊ-iÛiÀiÊ* Ê>iÀ}Þ\ÊÝyÝ>VÊ{ääÊ}Ê6Ê+Ó{
Late-onset VAP (all VAP that is not early-onset)
Etiology: S. aureus, P. aeruginosa, other Gram-negative bacilli
UÊÊ6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊPLUSÊQ*«iÀ>VÉ
tazobactam 4.5 g IV Q6H OR Cefepime 2 g IV OR +nRʱ Gentamicin
(see dosing section, p. 146)
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊPLUS
Q «ÀyÝ>VÊ{ääÊ}Ê6Ê+nÊ",ÊâÌÀi>ÊÓÊ}Ê6Ê+nRÊPLUS
Gentamicin (see dosing section, p. 146)
Enterococci and candida species are often isolated from sputum in
hospitalized patients. In general, they should be considered to be
colonizing organisms and should not be treated with antimicrobials.
If the patient is immunocompromised, consider adding Azithromycin
500 mg Q24H to Piperacillin/tazobactam, Cefepime or Aztreonam to
cover Legionella
Duration
UÊÊ3 days if CPIS remains ≤ 6 in patients with initial CPIS ≤ ÈÆÊ6*ÊÃÊ
unlikely
UÊÊ7 days if the patient has clinical improvement
UÊÊvÊÃÞ«ÌÃÊ«iÀÃÃÌÊ>ÌÊÇÊ`>ÞÃÊVÃ`iÀÊ>ÌiÀ>ÌÛiÊÃÕÀViÊ>`ÉÀÊ
bronchoscopy with quantitative cultures
UÊÊ6*Ê>ÃÃV>Ìi`ÊÜÌ ÊS. aureus bacteremia should be treated for at
least 14 days
89
6.12 Pulmonary infections
EMPIRIC TREATMENT
6.12 Pulmonary infections
TREATMENT NOTES
UÊÊTreatment MUST be narrowed based on culture results
UÊÊ/LÀ>ÞVÊÃÊÀiVi`i`Ê>ÃÊ>ÊÃiV`Ê>}iÌÊÌÊLÀ>`iÊi«ÀVÊ
coverage rather than fluoroquinolones because of high rates of
resistance to fluoroquinolones in the institution.
UÊÊÌVÀL>ÊÌ iÀ>«ÞÊÃ Õ`ÊLiÊÌ>Ài`ÊViÊÃÕÃVi«ÌLÌiÃÊ>ÀiÊ
known. Vancomycin should be stopped if resistant Gram-positive
organisms are not recovered. Gram-negative coverage can be
reduced to a single susceptible agent in most cases. The benefits of
combination therapy in the treatment of Pseudomonas are not well
`VÕiÌi`ÆÊvÊÌÊÃÊ`iÃÀi`]ÊÌ iÊVÃ`iÀÊ}Û}ÊÌÊvÀÊÌ iÊwÀÃÌÊÇÓÊ
hours of therapy only.
Diagnosis
UÊÊ6*ÊÃÊ`vwVÕÌÊÌÊ`>}Ãi°
UÊÊ >VÌiÀ>ÊÊi`ÌÀ>V i>ÊÃÕVÌÊ>ÞÊÀi«ÀiÃiÌÊÌÀ>V i>ÊVâ>ÌÊ
and NOT infection.
UÊÊ+Õ>ÌÌ>ÌÛiÊVÕÌÕÀiÃÊvÊ ÊyÕ`ÊV>Ê i«Ê`ÃÌ}ÕÃ ÊLiÌÜiiÊ
Vâ>ÌÊ>`ÊviVÌÆÊ≥ 104 cfu/ml is considered significant
growth.
Other considerations
UÊÊ/À>V i>ÊVâ>ÌÊvÊÀ>i}>ÌÛiÃÊ>`ÊS. aureus is not
eradicated even though lower airways are sterilized. Thus, posttreatment cultures in the absence of clinical deterioration (fever,
rising WBC, new infiltrates, worsening ventilatory status) are not
recommended.
UÊÊ>`iµÕ>ÌiÊÌ>ÊÌÀi>ÌiÌÊvÊ6*ÊÃÊ>ÃÃV>Ìi`ÊÜÌ Ê } iÀÊÀÌ>ÌÞÊ
(even if treatment is changed once culture results are known).
,iviÀiViÃ\
/-É -ÊÕ`iiÃÊvÀÊ*É6\Ê, ÊÓääxƣǣ\Înn°
V>ÊÀiëÃiÊÌÊ6*\Ê, ÊÓää£Æ£ÈÎ\£ÎÇ££ÎÇx°Ê
6*\ÊÀV ÊÌiÀÊi`ÊÓäääÆ£Èä\£ÓÈÈ°
\Ê iÃÌÊ£nÆ££Î\{£ÓÓä°
*-ÊÃVÀi\ÊÊ,iÛÊ,iëÀÊ ÃÊ££Æ£{Î\££Ó£q££Ó°Ê
iÌiÀ}ÊVÕÀÃiÊvÊÌ iÀ>«ÞÊÕÃ}Ê *-Ê-VÀi\ÊÊÊ,iëÀÊ ÀÌÊ >ÀiÊi`ÊÓäääÆÊ
£ÈÓ\xäxÊ>`ÊÌiÃÛiÊ >ÀiÊi`ÊÓää{ÆÊÎä\ÊÇÎxqÇÎn°
90
UÊÊ/ iÀ>«ÞÊÃ Õ`ÊLiÊL>Ãi`ÊÊVÕÌÕÀiÊ>`ÊÃÕÃVi«ÌLÌÞÊ`>Ì>ÊÜ iÊ
>Û>>LiÆÊÌ iÊ>}iÌÊÜÌ ÊÌ iÊ>ÀÀÜiÃÌÊëiVÌÀÕÊvÊ>VÌÛÌÞÊà Õ`ÊLiÊ
selected preferentially
UÊÊvÊ«ÃÃLi]ÊÃÌ«Êv>}Ê>ÌLÌVÃÊÜ iÊÌ>Ì}ÊiÜÊ>ÌLÌVÃ
UÊÊ} Ê`ÃiÃÊvÊ>ÌLÌVÃÊÃ Õ`ÊLiÊÕÃi`ÊÌÊ>ÝâiÊÕ}Ê«iiÌÀ>ÌÊ
and reduce the risk of emergence of resistance (see below)
TREATMENT NOTES FOR SPECIFIC ORGANISMS
UÊPseudomonas aeruginosa
UÊÊ*«iÀ>V]Ê ivi«i]Ê>`Ê ivÌ>â`iÊÃ Õ`ÊLiÊÕÃi`Ê
preferentially to Meropenem to minimize the induction of
resistance to beta-lactams by Meropenem
UÊÊ/ iÃiÊ>}iÌÃÊ>ÀiÊ}iiÀ>ÞÊVLi`ÊÜÌ Ê } `ÃiÊ
aminoglycosides based on in vitro evidence that there is synergy
against Pseudomonas
UÊÊÀÊ«>ÌiÌÃÊÜÌ Ê«iVÊ>iÀ}Þ]Ê «ÀyÝ>VÊÀÊâÌÀi>Ê
V>ÊLiÊVLi`ÊÜÌ Ê>Ê>}ÞVÃ`iÆÊ`iÃiÃÌâ>ÌÊÌÊLiÌ>
lactams or carbapenems should be strongly considered
UÊÊÊ«>ÌiÌÃÊÌiÀ>ÌÊÀÊÀiÃÃÌ>ÌÊÌÊ>}ÞVÃ`iÃ]Ê ÃÌÊV>Ê
be added
UÊÊ ÌÕÕÃÊvÕÃÊvÊLiÌ>>VÌ>ÃÊV>ÊLiÊVÃ`iÀi`ÊÊÃiÊ
«>ÌiÌÃÆÊÃiiÊ«°ÊÓnÊvÀÊÀiÊvÀ>Ì°
UÊÊ >i`Ê/LÀ>ÞVÊ>`Ê ÃÌÊV>ÊLiÊÕÃi`Ê>ÃÊ>`ÕVÌÛiÊÌ iÀ>«Þ
UÊStenotrophomonas maltophilia
UÊÊS. maltophilia isolated from sputum usually represents colonization.
UÊÊvÊÃÕ«iÀviVÌÊÃÊÃÕëiVÌi`]Ê/*É-8ÊÃÊÌ iÊwÀÃÌÊiÊ>}iÌ°Ê
UÊÊ/V>ÀVÉV>ÛÕ>>ÌiÊOR Minocycline may be used if susceptible in
«>ÌiÌÃÊÜ Ê>ÀiÊ>iÀ}VÊÀÊÌiÀ>ÌÊÀÊÀiÃÃÌ>ÌÊÌÊ/*É-8°Ê
UÊStaphylococcus aureus
UÊÊS. aureus isolated from sputum can indicate colonization or
infection.
UÊÊ7 iÌ iÀÊÌÀi>Ì}ÊVâ>ÌÊÜÌ ÊS. aureus in CF patients
improves outcomes is an area of active research, although
historically such colonization has not been successfully eradicated
with antimicrobial therapy. If this is attempted, possible agents
include Dicloxacillin, Cefazolin or Cephalexin for MSSA and
`>ÞV]Ê/*É-8]Ê ÝÞVÞVi]Ê>`ÊVÞViÊvÀÊ,-°ÊÊ
UÊÊ"Ý>VÊÃÊÌ iÊ`ÀÕ}ÊvÊV ViÊvÀÊ--Ê«iÕ>ÆÊ6>VÞVÊ
or Linezolid can be used for MRSA pneumonia.
91
6.12 Pulmonary infections
Antibiotic selection and dosing for cystic
fibrosis patients
6.12 Pulmonary infections
Antibiotic doses for cystic fibrosis infections – normal renal
function
UÊ ivÌ>â`i\ÊÓÊ}Ê6Ê+nÊ
UÊ*«iÀ>VÉÌ>âL>VÌ>\ÊΰÎÇxÊ}Ê6Ê+{
UÊ ivi«i\ÊÓÊ}Ê6Ê+n
UÊiÀ«ii\ÊÓÊ}Ê6Ê+n
UÊ «ÀyÝ>V\ÊÇxäÊ}Ê*"Ê+£ÓÊ",Ê{ääÊ}Ê6Ê+n
UÊâÌÀi>\ÊÓÊ}Ê6Ê+n
UÊ/V>ÀVÉV>ÛÕ>>Ìi\Êΰ£Ê}Ê6Ê+{
UÊ/*É-8ÊvÀÊS. maltophilia: 5 mg/kg IV/PO Q8H
UÊ/*É-8ÊvÀÊS. aureus: 2 DS tablets PO BID
UÊ ÃÌ\ÊÎÈÊ}É}É`>ÞÊ6Ê`Û`i`ÊÊÎÊ`ÃiÃÊ
UÊ >i`Ê/LÀ>ÞVÊ­/" ®®\ÊÎääÊ}Ê+£Ó
UÊ >i`Ê ÃÌ\ÊÇx£xäÊ}Ê+£ÓÊ`i«i`}ÊÊÌ iÊ`iÛiÀÞÊÃÞÃÌiÊÊ
Intravenous Tobramycin dosing and monitoring:
UÊ>`}Ê`Ãi\Ê£äÊ}É}É`>ÞÊ}ÛiÊÛiÀÊ£Ê ÕÀ°Ê
UÊÊ*i>ÊÃÊÀiVi`i`Ê>vÌiÀÊwÀÃÌÊ`Ãi]Ê£Ê ÕÀÊ>vÌiÀÊÌ iÊi`ÊvÊvÕÃÊ
ÜÌ Ê}>ÊvÊÓäÎäÊ>`ÊÌÀÕ} Ê>ÌÊÓÎÊ ÕÀÃÊÜÌ Ê}>ÊÊ£ÊV}É°Ê
UÊÊ ÃiÃÊV>ÊLiÊVÀi>Ãi`ÊÕ«ÊÌÊ£ÓÊ}É}É`>ÞÊvÊ>`iµÕ>ÌiÊ«i>ÃÊ
are not achieved. If trough is too low or too high, interval should be
changed.
92
Diagnosis
UÊÊ,iëÀ>ÌÀÞÊÛÀÕÃÊÌiÃÌ}Êà Õ`ÊLiÊLÌ>i`ÊÞi>ÀÊÀÕ`ÊÊ>ÞÊ«>ÌiÌÊ
for whom there is a clinical suspicion of respiratory virus infection. In
addition, during influenza and RSV season testing should be obtained
Ê«>ÌiÌÃÊÜÌ \
Ê UÊÊiÛiÀÊ>`ÊyÕiâ>iÊÃÞ«ÌÃÊ­ÃÀiÊÌ À>Ì]ÊÞ>}>]Ê>ÀÌ À>}>]Ê
cough, runny nose and/or headache)
Ê U Suspected bronchiolitis or pneumonia
Ê U COPD/asthma exacerbation or respiratory failure
Ê UÊ1iÝ«>i`Ê ÊiÝ>ViÀL>Ì
Ê UÊ `iÀÞÊ«>ÌiÌÃÊÜÌ ÊÕiÝ«>i`ÊiÜÊÃiÌÊ>>Ãi
Ê UÊ*Ài}>ÌÊ«>ÌiÌÃÊÜÌ ÊÕiÝ«>i`ÊÀiëÀ>ÌÀÞÊÃÞ«ÌÃ
Ê UÊÊ Ã«iVwVÊÃÞ«ÌÃÊ>`Ê>Ê`VÕiÌi`ÊiÝ«ÃÕÀiÊÌÊÃiiÊ
with a respiratory illness
UÊÊ,iëÀ>ÌÀÞÊÛÀÕÃÊÌiÃÌ}Ê>ÌÊÊ­iÊ *ÊyVi`ÊÃÜ>LÊà Õ`ÊLiÊ
submitted for either panel)
Ê UÊÊ/iÃÌ}ÊvÀÊÕV«iÌiÌÊ ÃÌÃ\ÊÀ>«`ÊÕViVÊ>V`ÊÌiÃÌÊvÀÊ,-6Ê
and influenza A/B
Ê UÊÊ/iÃÌ}ÊvÀÊÕV«ÀÃi`Ê ÃÌÃ]Ê«>ÌiÌÃÊLi}Ê>`ÌÌi`Ê
ÌÊÌ iÊ 1]Ê>`Ê«>ÌiÌÃÊÜÌ ÊÃÌÀÕVÌÕÀ>ÊÕ}Ê`Ãi>Ãi\ÊiÝÌi`i`Ê
panel for RSV, influenza A/B, adenovirus, human metapneumovirus,
parainfluenza 1-3, and rhinovirus
Treatment of influenza in inpatients
UÊÊ «ÀVÊÌÀi>ÌiÌÊvÊ>`ÕÌÊ«>ÌiÌÃÊÃ Õ`ÊLiÊVÃ`iÀi`ÊÊÌ iÊ
vÜ}ÊÃÌÕ>ÌÃÊ`ÕÀ}ÊyÕiâ>ÊÃi>Ã\Ê
Ê UÊÊ*>ÌiÌÃÊÜÌ ÊviÛiÀÊ>`ÊyÕiâ>iÊÃÞ«ÌÃ]ÊÕiÝ«>i`Ê
interstitial pneumonia or new respiratory failure without an obvious
non-influenza cause
UÊÊ/Ài>ÌiÌÊÃ Õ`ÊLiÊÌ>Ìi`ÊÊ>Ê«>ÌiÌÃÊÜ Ê>ÀiÊ>`ÌÌi`ÊÌÊÌ iÊ
hospital and have influenza with symptom onset in the past 48-72 hours
UÊÊ/ iÊÕÌÌÞÊvÊÌÀi>ÌiÌÊvÊ«>ÌiÌÃÊÜ Ê«ÀiÃiÌÊ>ÌiÊÊÌ iÊVÕÀÃiÊvÊ
disease is uncertain and the decision to treat these patients can be
made on a case-by-case basis
UÊÊÌÛÀ>ÊV ViÊÃÊ`i«i`iÌÊÊÌ iÊÃÕÃVi«ÌLÌÞÊvÊVÀVÕ>Ì}ÊÃÌÀ>ÃÊ
which may vary from season to season (see
www.hopkinsmedicine.org/amp for current recommendations)
UÊÊ ÕÀ>Ì\ÊxÊ`>ÞÃÊiÝVi«ÌÊvÀÊ«>ÌiÌÃÊÜÌ ÊÃ`ÊÀ}>ÊÌÀ>ë>Ì]Ê
hematologic malignancy, or BMT in whom 10 days can be given
because of prolonged viral shedding
93
6.13 Respiratory virus diagnosis and management
Respiratory virus diagnosis and management
6.13 Respiratory virus diagnosis and management
Infection control
UÊÊÊ`Û`Õ>ÃÊÜÌ ÊÃÕëiVÌi`ÊÀiëÀ>ÌÀÞÊÛÀÕÃÊviVÌÊà Õ`ÊLiÊ
placed on droplet precautions. A private room is required, unless
patients are cohorted. When outside of their room (i.e. during
transport) patients should wear a mask.
UÊÊÊ i>Ì ÊV>ÀiÊÜÀiÀÃÊÕÃÌÊÀiViÛiÊÌ iÊyÕiâ>ÊÛ>VViÊÞi>ÀÞ°
UÊÊ*iÀÃiÊÜÌ Ê`ÀiVÌÊ«>ÌiÌÊV>ÀiÊÀÊÜÀ}ÊÊVV>Ê>Ài>ÃÊÜ Ê >ÛiÊÌÊ
received the influenza vaccine are required to wear a mask when within 6
feet of a patient. The dates of the mask requirement are determined by
HEIC and based on influenza activity in the local community.
U No one with fever may work until at least 24 hours after fever
has resolved (without antipyretics). All personnel with respiratory
symptoms and fever must call or report to their supervisor and must
call Occupational Health Services (OHS).
UÊAfebrile employees who have respiratory systems must wear a
surgical mask during patient contact (≤ 6 ft).
UÊÊvÊ>ÊÕÛ>VV>Ìi`Ê 7ÊÃÊiÝ«Ãi`ÊÌÊ>Ê«>ÌiÌÊÜÌ Ê`VÕiÌi`Ê
influenza who was not on Droplet Precautions, notify HEIC and call
Occupational Health Services (OHS) immediately. OHS will decide
whether to recommend post-exposure prophylaxis.
Anti-influenza agents
Medication
Adult dosing
Side effects
Notes
Oseltamivir
Treatment:Ê
75 mg PO twice a day
vÀÊxÊ`>ÞÃÊ
Prophylaxis:Ê
75 mg PO once a day
\Ê>ÕÃi>]ÊÊ
vomiting
Ê
-iÛiÀi\
hypersensitivity,
neuropsychiatric
ÃiÊ>`ÕÃÌiÌÊ
needed for GFR
ÈäÊÉÊ
Treatment:Ê
10 mg (2 oral inhalations)
twice daily for 5 days
Prophylaxis:
10 mg (2 oral inhalations)
ViÊ>Ê`>ÞÊÊ
\Ê`>ÀÀ i>]ÊÊ
nausea, cough,
headache, and
dizziness
- Õ`Ê "/ÊLiÊÕÃi`Ê
in patients with
chronic underlying
airway diseases
Ê
<>>ÛÀÊ
Ê
94
Ê-iÛiÀi\ÊLÀV ë>Ã]Ê
hypersensitivity,
laryngeal edema,
facial swelling
Latent TB infection (LTBI)
UÊÊ*ÀiÛÕÃÊviVÌÊÜÌ ÊM. tuberculosis (MTB) that has been contained by
the host immune response
UÊÊ*>ÌiÌÊ>ÞÊ >ÛiÊ>Ê«ÃÌÛiÊÌiÃÌÊ­ÃiiÊLiÜ®ÊÀÊÃÕ}}iÃÌÛiÊÀ>`}À>« VÊ
findings such as calcified granulomata or minimal apical scarring, but do
not have symptoms of active TB disease
UÊÊ ÌÊviVÌÕÃÊ>`Ê`iÃÊÌÊÀiµÕÀiÊÃ>Ì
Tests to diagnose latent LTBI
UÊÊ Ì Ê/ÕLiÀVÕÊÃÊÌiÃÌÊ­/-/®Ê>`ÊÌiÀviÀÊ}>>ÊÀii>ÃiÊ>ÃÃ>ÞÊ­,®Ê
>ÀiÊ«iÀviVÌ]Ê>`Ê>ÞÊvviÀÊ`ÃVÀ`>ÌÊÀiÃÕÌÃÊ­HÓ䯮°ÊÊ-iÃÌÛÌÞÊvÊ/-/Ê
and IGRA are similar.
UÊÊ Ì ÊÌiÃÌÃÊà Õ`ÊLiÊÌiÀ«ÀiÌi`ÊÊÌ iÊVÌiÝÌÊvÊi«`i}VÊÀÃÊvÊ/ Ê
exposure
UÊÊ/ ÊÌ iÀ>«ÞÊÃ Õ`ÊÌÊLiÊÌ>Ìi`ÊÕÌÊ>VÌÛiÊ/ ÊÃÊiÝVÕ`i`Ê­LÞÊ
symptoms and radiography). Individuals with signs or symptoms of active
TB require further diagnostic workup before LTBI therapy.
UÊÊ/ ÊÌ iÀ>«ÞÊà Õ`ÊÌÊLiÊÃÌ>ÀÌi`ÊÊÌ iÊ Ã«Ì>ÊÜÌ ÕÌÊ>ÊVi>ÀÊvÜÕ«Ê
plan
Tuberculin skin test (TST)
UÊÊÌÀ>`iÀ>ÊiVÌÊvÊ«ÕÀwi`Ê«ÀÌiÊ`iÀÛ>ÌÛiÊ­** ®Ê>`Êi>ÃÕÀiiÌÊ
of induration diameter in 48-72
UÊÊÊ ÀÌiÀ>ÊvÀÊ>Ê«ÃÌÛiÊÌiÃÌÊ>Ài
UÊÊÊxÊÊqÊ } ÊÀÃÊvÊ`iÛi«}Ê>VÌÛiÊ/ Ê­i°}°]Ê6ÊviVÌ]ÊVÃiÊ
contact of TB case, immunocompromised)
UÊÊÊ£äÊÊqÊÌ iÀÊÀÃÊv>VÌÀÃÊvÀÊ/ ÊviVÌÊ­ 7]Ê 1]Ê ®
UÊÊÊ£xÊÊqÊÊÀÃÊv>VÌÀÃÊvÀÊ/
Interferon gamma release assay (IGRA)
UÊ,ÃÊi>ÃÕÀiÊÞ« VÞÌiÊÀii>ÃiÊvÊÌiÀviÀÊ}>>ÊÊÀiëÃiÊÌÊ
stimulation by MTB antigens.
UÊ,ÃÊ>ÀiÊiÃÃÊ>vviVÌi`ÊLÞÊ ÊÛ>VV>ÌÊÃÌ>ÌÕÃÊÀÊviVÌÊÜÌ ÊÃÌÊ
atypical mycobacteria (except M. marinum and M. kansasii) than TST
UÊ+Õ>ÌviÀ`/ÕLiÊ­+/®ÊÃÊÕÃi`Ê>ÌÊ°Ê,iÃÕÌÃÊ>ÀiÊÀi«ÀÌi`Ê>ÃÊ
positive, negative, or indeterminate. An indeterminate result means that the
test result is not valid, which can be due to errors in specimen collection
(most common--insufficient/incorrect shaking of tubes after blood draw
or processing delays), or associated with certain conditions such as HIV
with a low CD4 count, steroid use or other immunosuppression, and
>ÕÌÀÌÊQ>LÕÊΰxR°Ê`iÌiÀ>ÌiÊÀiÃÕÌÃÊvÌiÊÀiµÕÀiÊ>ÊÀi«i>ÌÊ
test (ensure proper specimen collection).
UÊ7 iÊ«ÀiÌiÃÌÊ«ÀL>LÌÞÊÀÊ«ÀiÛ>iViÊvÊ/ ÊÃÊx¯Ê­i°}°]Ê1-LÀÊ
ÜÌ ÕÌÊvÀi}ÊÌÀ>Ûi®]Ê**6ÊvÊ,ÊÃÊÀi`ÕVi`Ê­Çää¯]Ê°i°]Êv>Ãi«ÃÌÛiîÊ
Ü iÊ *6ÊÃÊ } Ê­¯®°ÊÊ
UÊ7 iÊ«ÀiÌiÃÌÊ«ÀL>LÌÞÊvÀÊviVÌÊÃÊ } Ê­i°}°]ÊvÀi}LÀ]ÊHÎä¯Ê/ Ê
«ÀiÛ>iVi®]Ê**6ÊvÊ,ÊVÀi>ÃiÃÊÌÊHx¯]ÊLÕÌÊ *6Ê`iVÀi>ÃiÃÊ
­nää¯]Ê°i°]Êv>Ãii}>ÌÛiî°ÊÊ
95
6.14 Tuberculosis (TB) infection
Tuberculosis (TB) infection
6.14 Tuberculosis (TB) infection
UÊ+Õ>ÌÌ>ÌÛiÊÀiÃÕÌÃÊ>ÞÊLiÊ i«vÕÊÌÊ}Õ`iÊÌiÀ«ÀiÌ>Ì°Ê Ã`iÀÊ Ê
VÃÕÌ>ÌÊvÀÊÀiÃÕÌÃÊi>ÀÊÌ iÊÌ Àià `ÊvÀÊ+/Ê«ÃÌÛi\Ê>Ì}i0.35.
Serial testing is not advised without ID consultation.
UÊ,ÃÊ`ÊÌÊ >ÛiÊ}`ÊÃiÃÌÛÌÞÊÀÊëiVwVÌÞÊvÀÊ`>}ÃÃÊvÊ>VÌÛiÊ/
Active TB infection
UÊÊVÌÛiÊÀi«V>ÌÊvÊ/ ÊV>ÕÃ}Ê«Õ>ÀÞÊÀÊiÝÌÀ>«Õ>ÀÞÊÃ}ÃÊÀÊ
symptoms
UÊÊ wÀi`ÊLÞÊ«ÃÌÛiÊ ÊÃi>À]Ê/ Ê`ÀiVÌÊÌiÃÌÊÀÊVÕÌÕÀi
UÊÊ,iµÕÀiÃÊ>ÀLÀiÊÃ>Ì
When to suspect active TB disease
High-risk individuals
UÊÊ,iViÌÊiÝ«ÃÕÀiÊÌÊ>Ê«iÀÃÊÜÌ ÊÜÊ/ ÆÊ ÃÌÀÞÊvÊ>Ê«ÃÌÛiÊ/-/ÆÊ
6ÊviVÌÆÊiVÌÊÀÊiVÌÊ`ÀÕ}ÊÕÃiÆÊvÀi}ÊLÀÌ ÊÀÊÀiÃ`iViÊ
Ê>ÊÀi}ÊÊÜ V Ê/ ÊV`iViÊÃÊ } ÆÊÀiÃ`iÌÃÊ>`Êi«ÞiiÃÊvÊ
} ÀÃÊV}Ài}>ÌiÊÃiÌÌ}ÃÊ­i°}°Ê«ÀÃîÆÊiLiÀà «ÊÊ>Êi`V>ÞÊ
Õ`iÀÃiÀÛi`]ÊÜViÊ««Õ>ÌÆÊ>Ì/ Ê>« >ÊÌ iÀ>«Þ
Clinical syndromes
UÊÊ Õ} ÊvÊ2 wk duration, with at least one additional symptom, including
fever, night sweats, weight loss, or hemoptysis
UÊÊÞÊÕiÝ«>i`ÊÀiëÀ>ÌÀÞÊiÃÃÊvÊ2 wk duration in a patient at high
risk for TB
UÊÊÞÊ«>ÌiÌÊÜÌ Ê6ÊviVÌÊ>`ÊÕiÝ«>i`ÊVÕ} Ê>`ÊviÛiÀÊ
UÊÊÞÊ«>ÌiÌÊÊ>Ì/ Ê>« >ÊÌ iÀ>«ÞÊÜÌ ÊÕiÝ«>i`ÊviÛiÀ
UÊÊ ÕÌÞ>VµÕÀi`Ê«iÕ>ÊÜ V Ê >ÃÊÌÊ«ÀÛi`Ê>vÌiÀÊÇÊ`>ÞÃÊvÊ
appropriate treatment
UÊÊV`iÌ>Êw`}ÃÊÊV iÃÌÊÀ>`}À>« ÊÃÕ}}iÃÌÛiÊvÊ/ Ê­iÛiÊvÊÃÞ«ÌÃÊ
are minimal or absent) in a patient at high risk for TB
Radiographic findings
UÊÊ*À>ÀÞÊ/ Ê­vÌiÊÕÀiV}âi`®\Ê >ÊÀiÃiLiÊ *Ê>`ÊÛÛiÊ>ÞÊLiÃÆÊ
>ÀÊ>`i«>Ì Þ]Ê«iÕÀ>ÊivvÕÃÃÊ>ÀiÊVÆÊV>ÛÌ>ÌÊÃÊÕV°Ê
`}ÃÊvÌiÊÀiÃÛiÊ>vÌiÀÊ£qÓÊÌ Ã°Ê/ iÃiÊ>ÀiÊVÊw`}ÃÊÊ
patients with advanced HIV infection and TB.
UÊÊ,i>VÌÛ>ÌÊ/ \ÊwÌÀ>ÌiÃÊÜÌ ÊÀÊÜÌ ÕÌÊV>ÛÌ>ÌÊÊÌ iÊÕ««iÀÊLiÃÊÀÊ
Ì iÊÃÕ«iÀÀÊÃi}iÌÃÊvÊÌ iÊÜiÀÊLiÃÆÊ >ÀÊ>`i«>Ì ÞÊÃÊÛ>À>LiÆÊ /Ê
ÃV>Ê>ÞÊ >ÛiʺÌÀiiLÕ`»Ê>««i>À>Vi°
Diagnosis
UÊÊ*>ÌiÌÃÊÜÌ ÊV >À>VÌiÀÃÌVÊÃÞ`ÀiÃÊ>`ÊÀ>`}À>« VÊw`}ÃÊÃ Õ`Ê
have expectorated sputum obtained for AFB smear and culture.
UÊÊ-iÃÌÛÌÞÊvÊ ÊÃi>ÀÊÊiÝ«iVÌÀ>Ìi`ÊëÕÌÕÊÃÊxäqÇä¯ÆÊÌÊÃÊ
ÜiÀÊÊ6³Ê«>ÌiÌðÊÀ}ÊiÝ«iVÌÀ>Ìi`ÊëÕÌÕ]Ê`ÕVi`ÊëÕÌÕ]Ê
bronchoscopy have higher sensitivity. AFB culture of lower respiratory tract
specimens is considered the gold standard.
UÊÊ ÊÃi>ÀÊ>`ÊVÕÌÕÀiÊÃ Õ`ÊLiÊLÌ>i`ÊÀi}>À`iÃÃÊvÊ 8,Ê
findings in patients with high clinical suspicion, HIV infection or other
ÕV«ÀÃi`ÊÃÌ>ÌiÃ°Ê 8,ÊÃÊÀ>ÊÊ>««ÀÝ>ÌiÞÊ£ä¯ÊvÊ6
infected patients with pulmonary TB.
96
Infection control
ÀLÀiÊ«ÀiV>ÕÌÃÊ>ÀiÊÀiµÕÀi`ÊÊÌ iÊvÜ}ÊV>ÃiÃ\
UÊÊ-ÕëVÊvÊ`Ãi>ÃiÊÃÕvwViÌÞÊ } ÊÌÊÜ>ÀÀ>ÌÊLÌ>}ÊëÕÌÕÊ Ê
smear/culture as described above
UÊÊ*ÃÌÛiÊ ÊÃi>ÀÊÀÊVÕÌÕÀiÊÕÌÊ`>}ÃÃÊvÊ/ ÊÛÃ°Ê /ÊÃÊVwÀi`
Algorithm for isolation when active TB is suspected
AIRBORNE PRECAUTIONS
IN NEGATIVE PRESSURE ROOM
Collect specimen(s) for AFB smear and culture
Expectorated sputum (3 required)*
Smear
positive
Mycobacterium
Tuberculosis
Direct Test (MTD)
automatically
performed
Induced sputum or bronchoscopy
Smear
negative
MTD
negative
Smear
positive
Obtain 2nd
and 3rd
specimen*
Smear
positive
MTD test
performed
MTD
positive
MTD
positive
Continue isolation until at
least 14 days of therapy
AND clinical improvement
AND 3 consecutive negative
smears (Call HEIC for
approval to D/C isolation on
smear positive patient.)
Smear
negative
If pt highly suspected
for TB, await culture
result and continue
isolation. Otherwise,
CALL HEIC 5-8384 to
DISCONTINUE ISOLATION
MTD
negative
CALL HEIC
5-8384 TO
DISCONTINUE
ISOLATION
*One expectorated sputum must be a first morning specimen; samples should
be collected at least 8 hours apart.
97
6.14 Tuberculosis (TB) infection
UÊÊ"LÌ>Ê>ÌÊi>ÃÌÊÎÊëÕÌÕÊëiViÃÊ­`ÕVi`ÊÀÊiÝ«iVÌÀ>Ìi`®ÊÜ iÊÌÀÞ}Ê
to diagnose TB in patients who are smear negative so as to increase the
chance of isolating the organism for diagnosis and susceptibility testing.
6.14 Tuberculosis (TB) infection
UÊÊÜÊ>VÌÛiÊ«Õ>ÀÞÊÀÊ>ÀÞ}i>Ê/ Ê­vÊ«>ÌiÌÊÃÊVÕÀÀiÌÞÊÊ/ Ê
treatment, consult with HEIC and patient’s local health department to obtain
treatment history in order to determine if infectious at the time of current
ëÌ>â>ÌÆÊÊi>ÌiÊ>ÀLÀiÊ«ÀiV>ÕÌÃÊ>ÀiÊÀiµÕÀi`®Ê
TREATMENT
Active TB
UÊ ÊVÃÕÌÊÃÊÃÌÀ}ÞÊÀiVi`i`Ê
UÊÊ/ iÀ>«ÞÊÃ Õ`ÊLiÊÌ>Ìi`ÊvÀÊ«>ÌiÌÃÊÜÌ Ê«ÃÌÛiÊ ÊÃi>ÀÊ>`ÊVV>Ê
findings consistent with active TB.
UÊÊ/ iÀ>«ÞÊÃ Õ`ÊLiÊVÃ`iÀi`ÊvÀÊ«>ÌiÌÃÊÜÌ Êi}>ÌÛiÊ ÊÃi>ÀÃÊ
when suspicion of TB is high and no alternate diagnosis exists. Multiple
specimens should be obtained for culture prior to treatment.
UÊÕÀÊ`ÀÕ}ÃÊ>ÀiÊiViÃÃ>ÀÞÊvÀÊÌ>Ê« >ÃiÊ­ÓÊÌ Ã®°Ê
UÊÃ>â`Ê­ ®ÊÎääIÊ}Ê­xÊ}É}®Ê*"Ê`>ÞÊ
UÊ,v>«Ê­,®ÊÈääIÊ}Ê­£äÊ}É}®Ê*"Ê`>Þ
UÊÊ*ÞÀ>â>`iÊ­*<®Ê£äääÊ}Ê*"Ê`>ÞÊ­{äqxxÊ}®Ê",Ê£xääÊ}Ê*"Ê
`>ÞÊ­xÈqÇxÊ}®Ê",ÊÓäääIÊ}Ê*"Ê`>ÞÊ­ÇÈqäÊ}®Ê
UÊÊ Ì >LÕÌÊ­ ®ÊnääÊ}Ê*"Ê`>ÞÊ­{äqxxÊ}®Ê",Ê£ÓääÊ}Ê*"Ê`>ÞÊ
­xÈqÇxÊ}®Ê",Ê£ÈääIÊ}Ê*"Ê`>ÞÊ­ÇÈqäÊ}®Ê
*Max dose regardless of weight.
UÊÊ*ÞÀ`ÝiÊÓxÊ}Ê*"Ê`>ÞÊÃÊÀiVi`i`ÊÌÊ«ÀiÛiÌÊ Ê>ÃÃV>Ìi`Ê
peripheral neuropathy in patients with HIV, malnutrition, alcohol abuse,
diabetes mellitus, renal failure or in pregnant or breastfeeding women.
Drug toxicity and monitoring
UÊÊÃ>â`\Ê>ÃÞ«Ì>ÌVÊiiÛ>ÌÊÊ i«>ÌVÊiâÞiÃ]ÊÃiÀÕÃÊ>`Êv>Ì>Ê
hepatitis, peripheral neurotoxicity
UÊÊ,v>«\ÊÀ>}iÊ`ÃVÀ>ÌÊvÊL`ÞÊyÕ`Ã]Ê i«>ÌÌÝVÌÞ]Ê«ÀÕÀÌÃÊÜÌ Ê
or without rash
UÊÊ*ÞÀ>â>`i\Ê i«>ÌÌÝVÌÞ]Ê}ÕÌÞÊ«Þ>ÀÌ À>}>]Ê>ÃÞ«Ì>ÌVÊ
hyperuricemia, acute gouty arthritis
UÊÊ Ì >LÕÌ\ÊÀiÌÀLÕL>ÀÊ>`Ê«iÀ« iÀ>ÊiÕÀÌÃÊÊ
U ÌÀ}\ÊL>ÃiiÊ i«>ÌVÊÌÀ>Ã>>ÃiÃ]ÊLÀÕL]Ê>>iÊ« ë >Ì>Ãi]Ê
creatinine and CBC are recommended for all adults initiating TB treatment.
Monthly hepatic panel is recommended for patients with baseline
abnormalities, history of liver disease or viral hepatitis, chronic alcohol
consumption, HIV, IVDU, pregnancy or immediate post-partum state or
those taking other potentially hepatotoxic medications. Therapy should
be discontinued immediately if AST and ALT are 3 times the upper limit
of normal (ULN) in the presence of jaundice or hepatitis symptoms or 5
times the ULN in the absence of symptoms.
,iviÀiViÃ\Ê
/-É -É
/-É -É
98
ÊÕ`iiÃÊvÀÊ`>}ÃÃÊvÊ/ \ÊÊÊ,iëÀÊ >ÀiÊi`ÊÓäääƣȣ\£ÎÇÈ°
ÊÕ`iiÃÊvÀÊÌÀi>ÌiÌÊvÊ/ \Ê7,ÆxÓ\,,££°Ê
6.15 Sepsis with no clear source
Sepsis with no clear source
NOTE: Refer to specific sections of these guidelines for empiric
treatment recommendations for specific sources of infection
EMPIRIC TREATMENT
Cultures MUST be sent to help guide therapy.
UÊÊQ*«iÀ>VÉÌ>âL>VÌ>IÊ{°xÊ}Ê6Ê+ÈÊ",Ê ivi«iIÊÓÊ}Ê6Ê+nRÊ
± Vancomycin (see dosing section, p. 150) (if at risk for MRSA) ±
Gentamicin (see dosing section, p. 146)
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\ÊQâÌÀi>ÊÓÊ}Ê6Ê+nÊ",Ê «ÀyÝ>VÊ{ääÊ
}Ê6Ê+nRÊPLUS Gentamicin (see dosing section, p. 146) PLUS
Vancomycin (see dosing section, p. 150)
*NOTE: If patient has history of ESBL-producing organism or has
suspected intra abdominal sepsis and recent prolonged exposure
( 7 days) to Piperacillin/tazobactam or Cefepime, substitute with
Meropenem 1 g IV Q8H.
Risk factors for MRSA
UÊÊ iÌÀ>ÊÛiÕÃÊV>Ì iÌiÀÊÊ«>Vi
UÊÊ"Ì iÀÊ`Üi}Ê >À`Ü>ÀiÊ
UÊÊÜÊVâ>ÌÊÜÌ Ê,-
UÊÊ,iViÌÊ­ÜÌ ÊÎÊÌ Ã®ÊÀÊVÕÀÀiÌÊ«À}i`Ê Ã«Ì>â>ÌÊ>
2 weeks
UÊÊ/À>ÃviÀÊvÀÊ>ÊÕÀÃ}Ê iÊÀÊÃÕL>VÕÌiÊv>VÌÞ
UÊÊiVÌÊ`ÀÕ}ÊÕÃi
TREATMENT NOTES
UÊÊÀÊ«>ÌiÌÃÊÜÌ ÊÀi>ÊÃÕvwViVÞÊÀÊ>}ÞVÃ`iÊÌiÀ>Vi]Ê>Ê
beta-lactam may be combined with a fluoroquinolone IF 2 agents are
needed.
UÊÊ*ÌiÌ>ÊÃÕÀViÃÊ­i°}°]Ê«iÕ>]Ê«iÀÌÌÃ]ÊiÌV°®Êà Õ`ÊLiÊ
considered when selecting therapy.
UÊÊ «ÀVÊÌ iÀ>«ÞÊÃÊ" 9Ê>««À«À>ÌiÊÜ iÊVÕÌÕÀiÃÊ>ÀiÊ«i`}Ê
(72 hours max).
UÊÊ6>VÞVÊÃ Õ`Ê>ÃÌÊ>Ü>ÞÃÊLiÊÃÌ««i`ÊvÊÊÀiÃÃÌ>ÌÊÀ>
positive organisms are recovered in cultures.
99
6.16 Skin, soft-tissue, and bone infections
Skin, soft-tissue, and bone infections
Cellulitis
UÊÊÜ>ÞÃÊiiÛ>ÌiÊ>vviVÌi`ÊiÝÌÀiÌÞ°Ê/Ài>ÌiÌÊv>ÕÀiÊÃÊÀiÊ
commonly due to failure to elevate than failure of antibiotics.
UÊÊ«ÀÛiiÌÊvÊiÀÞÌ i>ÊV>ÊÌ>iÊ`>ÞÃ]ÊiëiV>ÞÊÊ«>ÌiÌÃÊÜÌ Ê
lymphedema, because dead bacteria in the skin continue to induce
inflammation.
Non-suppurative cellulitis
Defined as cellulitis with intact skin and no evidence of purulent
drainage. Usually caused by beta-hemolytic streptococci (e.g. group A,
B, C, G streptococci) and MSSA.
TREATMENT
Oral (mild disease)
UÊÝVÉV>ÛÕ>>ÌiÊnÇxÊ*"Ê+£Ó
OR
UÊ i« >iÝÊxääÊ}Ê*"Ê+È
OR
UÊ* Ê>iÀ}Þ\Ê `>ÞVÊÎääÊ}Ê*"Ê+n
Parenteral (moderate to severe disease)
UÊ«VÉÃÕL>VÌ>Ê£°xÊ}Ê6Ê+È
OR
UÊ iv>âÊ£Ê}Ê6Ê+n
OR
UÊ* Ê>iÀ}Þ\Ê `>ÞVÊÈääÊ}Ê6Ê+n
Duration: 5-7 days
TREATMENT NOTES
UÊÊLiÌ> iÞÌVÊÃÌÀi«ÌVVVÊ>ÀiÊÃÕÃVi«ÌLiÊÌÊ«iV
UÊÊ `>ÞVÊÀiÃÃÌ>ViÊÃÊÃiiÊÊ£ÈÎίÊvÊ}ÀÕ«Ê ]Ê ]Ê>`ÊÊÃÌÀi«Ê
LÕÌÊÀi>ÃÊÜÊÊ}ÀÕ«ÊÊÃÌÀi«Ê­{qǯ®
UÊ ÕÀ>Ì\ÊxÇÊ`>ÞÃ
Suppurative cellulitis
Defined as cellulitis with purulent drainage or exudates in the absence of
a drainable abscess. Usually caused by S. aureus (MSSA and MRSA).
TREATMENT
Oral (mild disease)
UÊ/*É-8Ê£ÓÊ -ÊÌ>LÊ*"Ê OR
UÊ ÝÞVÞViÊ£ääÊ}Ê*"Ê Ê",ÊVÞViÊ£ääÊ}Ê*"Ê OR
UÊ `>ÞVÊÎääÊ}Ê*"Ê+n
100
Parenteral (moderate to severe disease)
UÊÊ6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®
Duration: 5-7 days
TREATMENT NOTES
UÊÊ,iÃÃÌ>ViÊÌÊyÕÀµÕiÃÊÊS. aureus is common and develops
µÕVÞÆÊÊx¯ÊvÊ,-ÊÃ>ÌiÃÊ>ÀiÊÀiÃÃÌ>ÌÊÌÊyÕÀµÕiðÊ
Monotherapy with fluoroquinolones for S. aureus infections is not
recommended.
UÊÊ,v>«ÊÃ Õ`Ê 6 ,ÊLiÊÕÃi`Ê>ÃÊÌ iÀ>«ÞÊLiV>ÕÃiÊÀiÃÃÌ>ViÊ
develops rapidly.
UÊÊ/ iÀiÊÃÊÊiÛ`iViÊÌ >ÌÊiâ`ÊÃÊÃÕ«iÀÀÊÌÊ/*É-8]Ê
Doxycycline, or Clindamycin in the management of skin infection or
osteomyelitis. Linezolid should only be considered when the S. aureus
isolate is resistant to or the patient is intolerant to these agents.
Less common causes of cellulitis
UÊÊ7Ì ÊLÕ>i]ÊÛiÃViÃ]Ê>`ÊÕViÀÃÊ>vÌiÀÊiÝ«ÃÕÀiÊÌÊÃi>Ü>ÌiÀÊÀÊÀ>ÜÊ
oysters, consider Vibrio vulnificus, especially in patients with liver
disease. Rare, but rapidly fatal if untreated. Treat with Ceftriaxone
1 g IV Q24H PLUS Doxycycline 100 mg PO BID.
UÊÊ iÕÌÀ«iV]ÊÃ`ÊÀ}>ÊÌÀ>ë>Ì]Ê>`ÊVÀÀ ÌVÊ«>ÌiÌÃÊ>ÞÊ
have cellulitis due to Gram-negative organisms. Consider expanding
coverage in these cases.
UÊÊvÊiÃV >À]ÊVÃ`iÀÊ>}Û>ÃÛiÊÀ}>ÃÃÊ­ ,]Ê>ëiÀ}ÃÃ]Ê`®°Ê
ID consult is recommended.
UÊÊ>Ê>`Ê Õ>ÊLÌiÃ\ÊPasteurella multocida should be covered in
cat and dog bites. Treat with Amoxicillin/clavulanate 875 mg PO BID
",Ê«VÉÃÕL>VÌ>Ê£°xqÎÊ}Ê6Ê+È°ÊvÊ* Ê>iÀ}Þ\ÊÝyÝ>VÊ
400 mg PO/IV Q24H.
Cutaneous abscess
UÊÊVÃÊ>`Ê`À>>}iÊ­E ®ÊÃÊÌ iÊ«À>ÀÞÊÌÀi>ÌiÌÊvÀÊ>ÊVÕÌ>iÕÃÊ
abscess.
UÊÊiÃÃÊÌ >ÌÊ>««i>ÀÊÃÕ«iÀwV>ÊV>ÊvÌiÊ >ÛiÊ>ÃÃV>Ìi`Ê>LÃViÃÃÊ
formation that is not clearly appreciated without debridement of the
wound or, on occasion, additional imaging.
UÊÊÌÊÌ iÊÌiÊvÊE ]Ê>ÊÃ>«iÊÃ Õ`ÊLiÊLÌ>i`ÊvÀÊVÕÌÕÀiÊ>`Ê
sensitivity testing.
UÊÊÃÌÊÃÌÕ`iÃÊÌ >ÌÊ >ÛiÊLiiÊ«ÕLà i`ÊÌÊ`>ÌiÊÃÕ}}iÃÌÊÌ >ÌÊ>ÌLÌVÃÊ
are adjunct to I&D in the management of uncomplicated skin
abscesses caused by CA-MRSA.
101
6.16 Skin, soft-tissue, and bone infections
OR
UÊ `>ÞVÊÈääÊ}Ê6Ê+nÊ­vÊ«>ÀiÌiÀ>ÊÌ iÀ>«ÞÊÃÊii`i`®
6.16 Skin, soft-tissue, and bone infections
UÊÊ`V>ÌÃÊvÀÊ>ÌVÀL>ÊÌ iÀ>«ÞÊÊ«>ÌiÌÃÊÜÌ ÊVÕÌ>iÕÃÊ
>LÃViÃÃiÃ\
UÊÊ-iÛiÀiÊÀÊÀ>«`ÞÊ«À}ÀiÃÃÛiÊviVÌÃ
UÊÊ/ iÊ«ÀiÃiViÊvÊiÝÌiÃÛiÊ>ÃÃV>Ìi`ÊViÕÌÃ
UÊ-}ÃÊ>`ÊÃÞ«ÌÃÊvÊÃÞÃÌiVÊiÃÃ
UÊÃÃV>Ìi`ÊÃi«ÌVÊ« iLÌÃ
UÊÊ >LiÌiÃÊÀÊÌ iÀÊÕiÊÃÕ««ÀiÃÃ
UÊ`Û>Vi`Ê>}i
UÊÊV>ÌÊvÊÌ iÊ>LÃViÃÃÊÊ>Ê>Ài>ÊÜ iÀiÊV«iÌiÊ`À>>}iÊÃÊ
difficult (e.g. face, genitalia)
UÊÊ>VÊvÊÀiëÃiÊÌÊVÃÊ>`Ê`À>>}iÊ>i
UÊÊ/ iÀ>«ÞÊÃ Õ`ÊLiÊ}ÛiÊbefore incision and drainage in patients with
prosthetic heart valves or other conditions placing them at high risk
for endocarditis.
EMPIRIC TREATMENT
If antibiotic treatment is thought to be necessary, regimens are the
same as for suppurative cellulitis above.
Management of recurrent MRSA skin infections
1. Education regarding approaches to personal and hand
hygiene
UÊÊ*À>VÌViÊvÀiµÕiÌÊ >`Ê Þ}iiÊÜÌ ÊÃ>«Ê>`ÊÜ>ÌiÀÊ>`ÉÀÊ
alcohol based hand gels, especially after touching infected skin or
wound bandages.
UÊÊ ÛiÀÊ`À>}ÊÜÕ`ÃÊÜÌ ÊVi>]Ê`ÀÞÊL>`>}iÃ
UÊÊ ÊÌÊà >ÀiÊ«iÀÃ>ÊÌiÃÊ­i°}°ÊÀ>âÀÃÆÊÕÃi`ÊÌÜiÃÊ>`ÊVÌ }Ê
before washing)
UÊÊ,i}Õ>ÀÊL>Ì }
UÊÊÛ`Ê>ÊÃ >Û}Ê
UÊÊ>Õ`iÀÊVÌ }]ÊÃ iiÌÃ]ÊÌÜiÃÊÊ ÌÌiÃÌÊÃÕÌ>LiÊÌi«iÀ>ÌÕÀi
UÊÊ i>Ê>Ê«iÀÃ>ÊëÀÌ}ÊVÌ }ÉiµÕ«iÌÊ
2. Decontamination of the environment
UÊÊ i>Ê } ÊÌÕV Ê>Ài>ÃÊÊÌ iÊL>Ì ÀÊÜÌ Ê>Ê`ÃviVÌ>ÌÊ>VÌÛiÊ
against S. aureusÊ`>ÞÊ­i°}°]Ê£ä¯Ê`ÕÌiÊLi>V ®°Ê
3. Topical decolonization (consider if a patient has ≥ 2 episodes
in 1 year or other household members develop infection)
UÊÊÕ«ÀVÊÌÜViÊ`>ÞÊvÀÊxÊ`>ÞÃÊ>ÞÊLiÊVÃ`iÀi`ÊÊ«>ÌiÌÃÊ
ÜÌ Ê`VÕiÌi`ÊiÛ`iViÊvÊ,-Ê>Ã>ÊVâ>ÌÆÊ
Mupirocin therapy should be initiated after resolution of acute
infection. Mupirocin should not be used in patients or patients’
family members who are not documented to have MRSA nasal
colonization.
102
NOTE: Data on efficacy and durability of the decontamination and
decolonization strategies described above are limited.
,iviÀiViÃ\
/*É-8ÊvÀÊ,-\ÊÊÌiÀÊi`Ê£ÓÆ££Ç\Îän°
-ÊÕ`iiÃÊvÀÊÌÀi>ÌiÌÊvÊ,-ÊviVÌÃ\Ê ÊviVÌÊ ÃÊÓ䣣ÆxÓ\£qÎn°Ê
Ì}ÞÊvÊÃÕ««ÕÀ>ÌÛiÊViÕÌÃ\Êi`ViÊÓä£äÆn\Ó£ÇqÓÓÈ°
Diabetic foot infections
EMPIRIC TREATMENT
Treatment depends on clinical severity
Infection Severity
Uninfected
Mild
Clinical Manifestations
No purulence or inflammation*
Presence of purulence and 1 sign of inflammation*
and cellulitis (if present) 2 cm around ulcer limited to
skin or superficial subcutaneous tissue
Moderate
Same as mild PLUSÊ>ÌÊi>ÃÌÊiÊvÊÌ iÊvÜ}\Ê 2
cm of cellulitis, lymphangitic streaking, spread beneath
the superficial fascia, deep tissue abscess, gangrene,
involvement of muscle, tendon, joint, or bone
Severe
Any of above PLUS systemic toxicity or metabolic
instability
*erythema, pain, tenderness, warmth, induration
MILD INFECTIONS
Oral regimens
UÊÊÝVÉV>ÛÕ>>ÌiÊnÇxÊ}Ê*"Ê OR
UÊÊ i« >iÝÊxääÊ}Ê*"Ê+
OR
UÊÊ `>ÞVÊÎääÊ}Ê*"Ê/ Ê­VÛiÀÃÊ,-®
Parenteral regimens
UÊÊ `>ÞVÊÈääÊ}Ê6Ê+nÊ­VÛiÀÃÊ,-®
OR
103
6.16 Skin, soft-tissue, and bone infections
UÊÊ >Ì }ÊÀÊÃ ÜiÀ}ÊÜÌ ÊV À iÝ`iÊÀÊ iÝ>V À« iÊ­ÀÊ
`ÕÌiÊLi>V ÊL>Ì Ã®ÊiÛiÀÞÊÌ iÀÊ`>ÞÊvÀÊ£ÊÜiiÊÌ iÊÌÜViÊÜiiÞÆÊ
do not get these substances into ears or eyes
UÊÊ-ÞÃÌiVÊ>ÌLÌVÃÊ>ÀiÊ "/ÊÀiVi`i`ÊÃiÞÊvÀÊ`iVâ>Ì
4. Evaluation of other family members
UÊÊÌÀ>v>ÞÊÌÀ>ÃÃÃÊà Õ`ÊLiÊ>ÃÃiÃÃi`Ê>`ÊvÊ«ÀiÃiÌ]Ê
all members should participate in hygiene and decolonization
strategies above, starting at that same time and after the acute
infection is controlled.
6.16 Skin, soft-tissue, and bone infections
UÊÊ"Ý>VÊ£ÓÊ}Ê6Ê+{
OR
UÊÊ iv>âÊ£Ê}Ê6Ê+n
MODERATE INFECTIONS
UÊÊ ÀÌ>«iiÊ£Ê}Ê+Ó{
OR
UÊÊQ «ÀyÝ>VIÊxääÊ}Ê*"Ê Ê",Ê «ÀyÝ>VIÊ{ääÊ}Ê6Ê+£ÓRÊ
PLUS ONEÊvÊÌ iÊvÜ}ÊQ `>ÞVÊÈääÊ}Ê6Ê+nÉÎääÊ}Ê*"Ê
/ Ê",ÊiÌÀ`>âiÊxääÊ}Ê6É*"Ê/ R
* BUT avoid fluoroquinolones in patients who were on them as
outpatients
If patient at risk for MRSA, add Vancomycin to regimens that do not
include Clindamycin.
Risk factors for MRSA
UÊÊÃÌÀÞÊvÊVâ>ÌÊÀÊviVÌÊÜÌ Ê,-
UÊÊ,iViÌÊ­ÜÌ ÊÎÊÌ Ã®ÊÀÊVÕÀÀiÌÊ«À}i`Ê Ã«Ì>â>ÌÊÊÓÊ
weeks
UÊÊ/À>ÃviÀÊvÀÊ>ÊÕÀÃ}Ê iÊÀÊÃÕL>VÕÌiÊv>VÌÞ
UÊÊiVÌÊ`ÀÕ}ÊÕÃi
SEVERE INFECTIONS
UÊÊ*«iÀVÉÌ>âL>VÌ>Ê{°xÊ}Ê6Ê+È
OR
UÊÊQ «ÀyÝ>VIÊ{ääÊ}Ê6Ê+nÊ",ÊâÌÀi>ÊÓÊ}Ê6Ê+nRÊPLUS
Clindamycin 600 mg IV Q8H
* Avoid fluoroquinolones in patients who were on them as outpatients.
If patient at risk for MRSA (see above)
UÊÊ*«iÀ>VÉÌ>âL>VÌ>Ê{°xÊ}Ê6Ê+ÈÊPLUS Vancomycin (see dosing
section, p. 150)
OR
UÊÊQ «ÀyÝ>VIÊ{ääÊ}Ê6Ê+nÊ",ÊâÌÀi>ÊÓÊ}Ê6Ê+nRÊPLUS
Metronidazole 500 mg IV Q8H PLUS Vancomycin (see dosing section,
p. 150)
* Avoid fluoroquinolones in patients who were on them as outpatients
TREATMENT NOTES
Management
UÊÊÊÕÌ`ÃV«>ÀÞÊ>««À>V ÊÌÊ>>}iiÌÊÃ Õ`ÊVÕ`iÊÜÕ`Ê
care consultation, assessment of vascular supply, vascular and/or
general surgery consultation and infectious diseases consultation.
UÊÊ Ã`iÀÊiVÀÌâ}Êv>ÃVÌÃÊÊ«>ÌiÌÃÊÜ Ê>ÀiÊÃiÛiÀiÞÊ°
UÊÊÌLÌVÊÌ iÀ>«ÞÊà Õ`ÊLiÊ>ÀÀÜi`ÊL>Ãi`ÊÊVÕÌÕÀiÊÀiÃÕÌð
104
Diagnosis
UÊÊ ÕÌÕÀiÃÊvÊÌ iÊÕViÀÊL>ÃiÊ>vÌiÀÊ`iLÀ`iiÌÊV>Ê i«Ê}Õ`iÊÌ iÀ>«Þ°Ê
Biopsy of unexposed bone is NOT recommended. Avoid swabbing
non-debrided ulcers or wound drainage.
UÊÊ1ViÀÊyÀÊà Õ`ÊLiÊ«ÀLi`ÊV>ÀivÕÞ°ÊvÊLiÊV>ÊLiÊÌÕV i`ÊÜÌ Ê>Ê
metal probe then the patient should be treated for osteomyelitis with
antibiotics in addition to surgical debridement.
UÊÊ*>Ì>ÀÊv>ÃVÌÃÊ>`Ê>Ê`ii«ÊvÌë>ViÊviVÌÊV>ÊLiÊ«ÀiÃiÌ°Ê
Consider imaging to look for deep infections.
UÊÊ*ÕÌÀ`Ê`ÃV >À}iÊÃÊ`>}ÃÌVÊvÊÌ iÊ«ÀiÃiViÊvÊ>>iÀLið
UÊÊÊ,ÊÃÊÀiÊÃiÃÌÛiÊ>`ÊëiVwVÊÌ >ÊÌ iÀÊ`>ÌiÃÊvÀÊ`iÌiVÌÊ
of soft-tissue lesions and osteomyelitis.
Duration
UÊÊ ÕÀ>ÌÊvÊÌÀi>ÌiÌÊÜÊ`i«i`ÊÊÀ>«`ÌÞÊvÊÀiëÃiÊ>`Ê
presence of adequate blood supply.
UÊÊiÞÊii`ÊÃ ÀÌiÀÊÌÀi>ÌiÌÊÜÌ Ê>`iµÕ>ÌiÊÃÕÀ}V>ÊÌiÀÛiÌÊ
­Çq£äÊ`>ÞÃÊ«ÃÌ«®Ê>`Ê}iÀÊvÀÊÃÌiÞiÌð
UÊÊ >}iÊÌÊÀ>ÊÀi}iÊÜ iÊ«>ÌiÌÊÃÊÃÌ>Li°
,iviÀiVi\
-ÊÕ`iiÃÊvÀÊ`>LiÌVÊvÌÊviVÌ°Ê ÊviVÌÊ ÃÊÓä£ÓÆx{\£ÎÓ£Çΰ
Surgical-site infections (SSI)
EMPIRIC TREATMENT
Infections following clean procedures (e.g. orthopedic joint
replacements, open reduction of closed fractures, vascular procedures,
median sternotomy, craniotomy, breast and hernia procedures)
UÊÊ"Ý>VÊ£qÓÊ}Ê6Ê+{
OR
UÊÊ iv>âÊ£Ê}Ê6Ê+n
OR
105
6.16 Skin, soft-tissue, and bone infections
Microbiology
UÊÊ iÕÌÃÊÜÌ ÕÌÊ«iÊÜÕ`ÊÀÊviVÌi`ÊÕViÀ]Ê>ÌLÌVÊ>Ûi\Ê
beta-hemolytic streptococci, S. aureus
UÊÊviVÌi`ÊÕViÀ]ÊV ÀVÊÀÊ«ÀiÛÕÃÞÊÌÀi>Ìi`ÊÜÌ Ê>ÌLÌVÃ\ÊS. aureus,
beta-hemolytic streptococci, Enterobacteriaceae
UÊÊ Ý«ÃÕÀiÊÌÊÃ>}]ÊÜ À«]Ê ÌÊÌÕL\ÊÕÃÕ>ÞÊ«ÞVÀL>]Ê>ÞÊ
involve Pseudomonas
UÊÊ ÀVÊÜÕ`ÃÊÜÌ Ê«À}i`ÊiÝ«ÃÕÀiÊÌÊ>ÌLÌVÃ\Ê>iÀLVÊÀ>
positive cocci (GPC), Diphtheroids, Enterobacteriaceae, other Gramnegative rods (GNR) including Pseudomonas
UÊÊ iVÀÃÃÊÀÊ}>}Àii\ÊÝi`Ê>iÀLVÊ* Ê>`Ê ,]Ê>>iÀLiÃ
6.16 Skin, soft-tissue, and bone infections
UÊÊ* Ê>iÀ}Þ\Ê `>ÞVÊÈääÊ}Ê6Ê+n
OR
UÊÊÛÛiiÌÊvÊ >À`Ü>ÀiÊÀÊ,-ÊÃÕëiVÌi`\Ê6>VÞVÊ
(see dosing section, p. 150)
Exception: Saphenous vein graft harvest site infections should be
treated with Ertapenem 1 g IV Q24H
Infections following contaminated procedures (GI/GU procedures,
oropharyngeal procedures, obstetrical and gynecology procedures)
Patients not on broad-spectrum antibiotics at time of surgery and
not severely ill
UÊÊ ÀÌ>«iiÊ£Ê}Ê6Ê+Ó{
OR
UÊÊ* Ê>iÀ}Þ\ÊQ «ÀyÝ>VÊxääÊ}Ê*"Ê Ê",Ê «ÀyÝ>VÊ{ääÊ}Ê
6Ê+£ÓRÊPLUS Clindamycin 600 mg IV Q8H
Patients on broad-spectrum antibiotics at time of surgery or
severely ill
UÊÊ*«iÀ>VÉÌ>âL>VÌ>ÊΰÎÇxÊ}Ê6Ê+ÈÊ´Ê6>VÞVÊ
(see dosing section, p. 150) (if hardware present or MRSA suspected)
OR
UÊÊ ÃiÛiÀiÊ* Ê>iÀ}Þ\Ê ivi«iÊ£Ê}Ê6Ê+nÊPLUS Metronidazole
xääÊ}Ê6Ê+nÊ´Ê6>VÞVÊ­ÃiiÊ`Ã}]Ê«°Ê£xä®Ê­vÊ >À`Ü>ÀiÊ
present or MRSA suspected)
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊPLUS
Q «ÀyÝ>VÊ{ääÊ}Ê6Ê+nÊ",ÊâÌÀi>ÊÓÊ}Ê6Ê+nRÊPLUS
Metronidazole 500 mg IV/PO Q8H
Deep fascia involvement
UÊÊ/Ài>ÌÊ>ÃÊiVÀÌâ}Êv>ÃVÌÃÊ­ÃiiÊÃÕLÃiµÕiÌÊÃiVÌ®
TREATMENT NOTES
Microbiology
UÊÊÜ}ÊVi>Ê«ÀVi`ÕÀiÃÊ­ÊiÌÀÞÊvÊÉ1ÊÌÀ>VÌî
UÊÊStaphylococcus aureus
UÊÊ-ÌÀi«ÌVVV]Ê}ÀÕ«ÊÊ­iëiV>ÞÊÜÌ Êi>ÀÞÊÃiÌ]ÊÊÇÓÊ ÕÀî
UÊÊ >}Õ>Ãii}>ÌÛiÊÃÌ>« ÞVVV
UÊÊÜ}ÊVi>VÌ>>Ìi`Ê>`ÊVÌ>>Ìi`Ê«ÀVi`ÕÀiÃÊ­iÌÀÞÊvÊ
GI/GU tracts with or without gross contamination)
UÊÊ"À}>ÃÃÊ>LÛi
UÊÊÀ>i}>ÌÛiÊÀ`Ã
UÊÊ>iÀLiÃÊ­VÃ`iÀÊClostridiaÊë«°ÊÊi>ÀÞÃiÌÊviVÌ]Ê£qÓÊ
days)
106
Other management issues
UÊÊ>ÞÊ>`ÛV>ÌiÊÌ >ÌÊÊviVÌi`ÊÜÕ`ÃÊLiÊiÝ«Ài`ÊLÌ ÊÌÊ`iLÀ`iÊ
and to assess depth of involvement.
UÊÊ-Õ«iÀwV>ÊviVÌÃÊ>ÞÊLiÊ>`iµÕ>ÌiÞÊÌÀi>Ìi`ÊÜÌ Ê`iLÀ`iiÌÊ
alone.
UÊÊ ii«iÀÊviVÌÃÊ­ViÕÌÃ]Ê«>VÕÌîÊii`Ê>`ÕVÌÛiÊ>ÌLÌVð
UÊÊviVÌÃÊÌ >ÌÊiÝÌi`ÊÌÊÌ iÊv>ÃV>ÊÃ Õ`ÊLiÊ>>}i`Ê>ÃÊiVÀÌâ}Ê
fasciitis.
UÊÊ*>ÌiÌÃÊÜÌ Ê Þ«ÌiÃÊà Õ`Ê >ÛiÊÌ iÀÊÜÕ`ÃÊiÝ«Ài`ÊiÛiÊvÊ
they are unremarkable on physical exam.
Serious, deep-tissue infections (necrotizing fasciitis)
THESE ARE SURGICAL EMERGENCIES!
ANTIBIOTICS ARE ONLY AN ADJUNCT TO PROMPT
DEBRIDEMENT!
ID should also be consulted
EMPIRIC TREATMENT (adjunct to surgery)
UÊÊ6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊPLUSÊQ*«iÀ>VÉÊ
Ì>âL>VÌ>ÊΰÎÇxÊ}Ê6Ê+ÈÊ",Ê ivi«iÊ£Ê}Ê6Ê+nRÊPLUS
Clindamycin 600-900 mg IV Q8H
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊPLUS
Q «ÀyÝ>VÊ{ääÊ}Ê6Ê+nÊ´ÊiÌ>VÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê
«°Ê£{È®RÊPLUS Clindamycin 600-900 mg IV Q8H
TREATMENT NOTES
Conventional nomenclature and microbiology
Pyomyositis
UÊÊS. aureus most commonly
UÊÊ ÃÌÀ`>ÊÞiVÀÃÃÊqÊClostridia spp. (esp. C. perfringens)
UÊÊÀÕ«ÊÊÃÌÀi«ÌVVV>ÊÞiVÀÃÃ
107
6.16 Skin, soft-tissue, and bone infections
UÊÊiiÀ>Þ]Êi«ÀVÊÕÃiÊvÊ6>VÞVÊÃÊÌÊ`V>Ìi`ÊLiV>ÕÃiÊÌ iÊ
percentage of SSIs caused by MRSA is low at Johns Hopkins Hospital
­£äqÓ䯮
Risk factors for MRSA
UÊÃÌÀÞÊvÊVâ>ÌÊÀÊviVÌÊÜÌ Ê,-
UÊÊ,iViÌÊ­ÜÌ ÊÎÊÌ Ã®ÊÀÊVÕÀÀiÌÊ«À}i`Ê Ã«Ì>â>ÌÊÓÊ
weeks
UÊÊ/À>ÃviÀÊvÀÊ>ÊÕÀÃ}Ê iÊÀÊÃÕL>VÕÌiÊv>VÌÞ
UÊÊiVÌÊ`ÀÕ}ÊÕÃi
6.16 Skin, soft-tissue, and bone infections
Fasciitis
UÊÊ/Þ«iÊ£ÊqÊ*ÞVÀL>ÊviVÌÃÊÜÌ Ê>>iÀLiÃ]ÊÃÌÀi«ÌVVVÊ>`Ê
Gram-negative rods (Fournier’s gangrene is a type 1 necrotizing
fasciitis of the perineum)
UÊÊ/Þ«iÊÓÊqÊÀÕ«ÊÊÃÌÀi«ÌVVVÊ«Ài`>Ìi
UÊÊ >ÃiÃÊvÊv>ÃVÌÃÊV>ÕÃi`ÊLÞÊVÕÌÞ>ÃÃV>Ìi`Ê,-ÊÃÌÀ>ÃÊ >ÛiÊ
been reported
Diagnosis
UÊÊ >ÊLiÊ`vwVÕÌÊqÊ}>ÃÊ«À`ÕVÌÊÃÊÌÊÕÛiÀÃ>Ê>`ÊÃÊ}iiÀ>ÞÊ
absent in streptococcal diseases.
UÊÊ>Ì>Ê } Ê`iÝÊvÊÃÕëVÊÜ i\
UÊÊ*>ÌiÌÃÊ>ÀiÊÛiÀÞÊÊvÀÊViÕÌÃÊ­ Þ«ÌiÃ]ÊÌÝVÊ>««i>À>Vi®
UÊÊ*>ÊÕÌÊvÊ«À«ÀÌÊÌÊ« ÞÃV>Êw`}Ã
UÊÊiÃÌ iÃ>ÊÛiÀÊ>vviVÌi`Ê>Ài>
UÊÊ,ÃÊv>VÌÀÃÊÃÕV Ê>ÃÊ`>LiÌiÃ]ÊÀiViÌÊÃÕÀ}iÀÞÊÀÊLiÃÌÞ
UÊÊ`}ÃÊÃÕV Ê>ÃÊÃÊiVÀÃÃÊÀÊLÕ>i
UÊÊ*ÕÌÀ`Ê`ÃV >À}iÊÜÌ ÊÌ ]ʺ`à Ü>ÌiÀ»Ê«ÕÃ
UÊÊ /ÊÃV>ÊV>Ê i«ÊÜÌ Ê`>}ÃÃÊLÕÌÊvÊÃÕëVÊÃÊ`iÀ>ÌiÊÌÊ } ]Ê
surgical exploration is the preferred diagnostic test. DO NOT delay
surgical intervention to obtain CT.
,iviÀiVi\
-Ê}Õ`iiÃÊvÀÊ--/\Ê ÊviVÌÊ ÃÊÓääxÆÊ{£\£ÎÇÎq{äÈ°
Vertebral osteomyelitis, diskitis, epidural abscess
NOTE: In absence of bacteremia, clinical instability, or signs and
symptoms of spinal cord compromise strong consideration should be
given to withholding antibiotics until samples of abscess or bone can be
obtained for Gram-stain and culture.
EMPIRIC TREATMENT
UÊÊ6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®Ê±ÊQ ivÌÀ>ÝiÊÓÊ}Ê+£ÓÊOR
ivi«iÊÓÊ}Ê6Ê+nRÊ
OR
UÊÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®Ê±
Ciprofloxacin 400 mg IV Q8H
UÊ >ÀÀÜÊÌ iÀ>«ÞÊL>Ãi`ÊÊVÕÌÕÀiÊÀiÃÕÌð
TREATMENT NOTES
Microbiology
UÊÀ>«ÃÌÛiÊVVVÊÊÇx¯ÊvÊV>ÃiÃÊÜÌ Ê>ÀÌÞÊS. aureus
UÊÀ>i}>ÌÛiÊÀ`ÃÊÊH£ä¯
108
Duration
UÊ «`ÕÀ>Ê>LÃViÃÃÊÜÌ ÕÌÊÃÌiÞiÌÃ\Ê{qÈÊÜiiÃÊ
UÊ6iÀÌiLÀ>ÊÃÌiÞiÌÃʱÊi«`ÕÀ>Ê>LÃViÃÃ\ÊÈq£ÓÊÜiiÃÊ
UÊÊÊ«>ÌiÌÃÊÜÌ Ê >À`Ü>ÀiÊ«ÀiÃiÌÊ«À}i`ÊÀ>ÊÃÕ««ÀiÃÃÛiÊÌ iÀ>«ÞÊ
ÃÊ}iiÀ>ÞÊÀiµÕÀi`Ê>vÌiÀÊV«iÌÊvÊ6Ê>ÌLÌVÃÆÊÌ iÃiÊ`iVÃÃÊ
should be made in consultation with infectious diseases.
,iviÀiViÃ\Ê
-«>Êi«`ÕÀ>Ê>LÃViÃÃ\Ê Ê }ÊÊi`ÊÓääÈÆÎxx\Óä£ÓqÓä°Ê
-«>Êi«`ÕÀ>Ê>LÃViÃÃ\Ê+ÊÊi`ÊÓäänÆ£ä£\£q£Ó°Ê
109
6.16 Skin, soft-tissue, and bone infections
Management
UÊÊ"LÌ>ÊÌÜÊÃiÌÃÊvÊL`ÊVÕÌÕÀiÃ]Ê -,]Ê>`Ê ,*Ê«ÀÀÊÌÊÃÌ>ÀÌ}Ê
antibiotic therapy.
UÊÊÃÌÊÌÀ>ÛiÕÃÊ`ÀÕ}ÊÕÃiÀÃÊ>`Ê«>ÌiÌÃÊÜÌ ÕÌÊÃ}wV>ÌÊ
co-morbidities do not require empiric coverage for Gram-negative
rods.
UÊÊ «ÀVÊÀ>i}>ÌÛiÊVÛiÀ>}iÊÃ Õ`ÊLiÊÕÃi`ÊÊ«>ÌiÌÃÊÜÌ Ê`>LiÌiÃ]Ê
hardware in place or recent surgery, and recurrent urinary tract infections.
UÊ,ÊÜÌ ÊVÌÀ>ÃÌÊÃÊÌ iÊ>}}ÊiÌ `ÊvÊV Vi°
UÊÊvÊL`ÊVÕÌÕÀiÃÊ>ÀiÊi}>ÌÛiÊ /Ê}Õ`i`Êii`iÊL«ÃÞÉ>ëÀ>ÌÊ
should be obtained for Gram stain and cultures.
UÊÊ iÀ}iÌÊÃÕÀ}V>ÊVÃÕÌ>ÌÊÃÊÀiVi`i`ÊvÀÊ«>ÌiÌÃÊÜÌ Ê
signs and symptoms of spinal cord compromise.
UÊÊ-ÕÀ}V>ÊÌ iÀ>«ÞÊÃÊ«ÀiviÀÀi`ÊÊ>ÞÊV>ÃiÃÊvÊi«`ÕÀ>Ê>LÃViÃÃÉÊ
osteomyelitis (e.g. extensive infection, pre-vertebral abscess, spine
instability, hardware involvement). CT-guided aspiration and/or
antibiotic therapy alone may be considered in some circumstances.
Discussion with infectious diseases and surgery is recommended to
optimize management.
UÊÊ*>ÌiÌÃÊÃ Õ`Ê >ÛiÊvÀiµÕiÌÊ>ÃÃiÃÃiÌÊvÊiÕÀ}VÊvÕVÌ]Ê
particularly at the time of initial presentation.
UÊÊÊ«>ÌiÌÃÊÀiµÕÀiÊÌÀ}ÊvÀÊ>`iµÕ>ÌiÊÀiëÃiÊÌ ÀÕ} ÕÌÊÌ iÊ
ÌÀi>ÌiÌÊVÕÀÃiÆÊ ÊvÜÊÕ«Ê } ÞÊÀiVi`i`°Ê
110
Bacterial urinary tract infections (UTI)
Empiric treatment
ÊÌÀi>ÌiÌÊÕiÃÃÊÌ iÊ«>ÌiÌÊÃ\
UÊ*Ài}>ÌÊ
UÊÊLÕÌÊÌÊÕ`iÀ}Ê>ÊÕÀ}VÊ«ÀVi`ÕÀiÊ
UÊ*ÃÌÊÀi>ÊÌÀ>ë>Ì
UÊ iÕÌÀ«iV
1V«V>Ìi`\
UÊÊ ÌÀvÕÀ>ÌÊ­>VÀL`®) 100 mg PO Q12H for
xÊ`>ÞÃÊ­ "/ÊÊ«>ÌiÌÃÊÜÌ Ê À ÊxäÊÉ®
OR
UÊÊ i« >iÝÊxääÊ}Ê*"Ê+ÈÊvÀÊxÊ`>ÞÃÊ
OR
UÊÊ iv«`ÝiÊ£ääÊ}Ê*"Ê+£ÓÊvÀÊxÊ`>ÞÃÊ
OR
UÊÊ iv`ÀÊÎääÊ}Ê*"Ê+£ÓÊvÀÊxÊ`>ÞÃÊ
OR
UÊÊÊ/*É-8Ê£Ê -ÊÌ>LÊ*"Ê+£ÓÊvÀÊÎÊ`>ÞÃ
OR
UÊÊ6Ê«Ì\Ê iv>âÊ£Ê}Ê6Ê+nÊvÀÊÎÊ`>ÞÃ
«V>Ìi`\
UÊÊ->iÊÀi}iÃÊ>ÃÊ>LÛiÊiÝVi«ÌÊ`ÕÀ>ÌÊÃÊ
Çq£{Ê`>ÞÃ
Definition
Positive urine culture 100,000 CFU/mL
with no signs or symptoms
Signs and symptoms (e.g. dysuria, urgency
frequency, suprapubic pain)
AND pyuria (>10 WBC/hpf )
AND positive urine culture 100,000
CFU/mL
UÊÊUncomplicated: female, no urologic
abnormalities, no stones, no catheter
UÊÊComplicated: male gender, possible
stones, urologic abnormalities, pregnancy
Category
Asymptomatic
bacteriuria
Acute cystitis
UÊÊ1/ÃÊÊiÊ>ÀiÊÌÀ>`Ì>ÞÊVÃ`iÀi`ÊV«V>Ìi`°Ê
UTIs in men in the absence of obstructive pathology
(e.g. BPH, stones, strictures) are uncommon. Please
critically evaluate your diagnosis of UTI in male patients.
UÊÊ"À>ÊÌ iÀ>«ÞÊÃÊ«ÀiviÀÀi`Ê>`ÊÃ Õ`ÊLiÊ}ÛiÊÕiÃÃÊ
patient is unable to tolerate oral therapy
UÊÊvÊ6ÊLiÌ>>VÌ>ÃÊ>ÀiÊÕÃi`Êi«ÀV>ÞÊvÀÊÎÊ`>ÞÃ]ÊÊ
additional therapy is needed for uncomplicated cystitis
UÊÊvÊ6ÊLiÌ>>VÌ>ÃÊ>ÀiÊÕÃi`Êi«ÀV>ÞÊvÀÊÎÊ`>ÞÃÊ
or treating complicated cystitis, the patient can be
switched to an appropriate oral beta-lactam and duration
of IV therapy should be counted towards total duration
of therapy
UÊÊ"À>ÊÃvÞVÊV>ÊLiÊÕÃi`ÊvÊÃÕÃVi«ÌLiÊvÀÊÀ>
negative MDR organisms (susceptibilities must be
requested)
Notes
UÊÊ"LÌ>}ÊÀÕÌiÊVÕÌÕÀiÃÊÊ>ÃÞ«Ì>ÌVÊ«>ÌiÌÃÊÃÊ
not recommended
UÊÊÌLÌVÃÊ`ÊÌÊ`iVÀi>ÃiÊ>ÃÞ«Ì>ÌVÊL>VÌiÀÕÀ>ÊÀÊ
prevent subsequent development of UTIs
UÊÊÊ/ iÊ«ÀiÛ>iViÊvÊ>ÃÞ«Ì>ÌVÊL>VÌiÀÕÀ>ÊÃÊ
} \Ê£¯x¯ÊÊ«Àii«>ÕÃ>ÊÜi]Êί¯ÊÊ
«ÃÌi«>ÕÃ>ÊÜi]Ê{ä¯xä¯ÊÊ}ÌiÀÊV>ÀiÊ
ÀiÃ`iÌÃÊ>`ʯÓǯÊÊÜiÊÜÌ Ê`>LiÌið
NOTE: Ciprofloxacin is not recommended for empiric treatment for in-patients with non-catheter associated UTI at JHH due to the low rate of E. coli
ÃÕÃVi«ÌLÌÞÊ­Ç£¯®°Ê
Management of patients WITHOUT a urinary catheter
6.17 Urinary tract infections
111
Definition
Signs and symptoms (e.g. fever, flank pain)
AND pyuria
AND positive urine culture 100,000
CFU/mL
Many patients will have other evidence of
upper tract disease (i.e. leukocytosis,
WBC casts, or abnormalities upon imaging)
SIRS with urinary source of infection
Category
Acute
pyelonephritis
Urosepsis
Empiric treatment
UÊÊ ivÌÀ>ÝiÊ£Ê}Ê6Ê+Ó{
OR
UÊÊ ÀÌ>«iiÊ£Ê}Ê6Ê+Ó{Ê­vÊ ÃÌÀÞÊvÊ - ®
OR
UÊÊ* Ê>iÀ}Þ\ÊâÌÀi>Ê£Ê}Ê6Ê+nÊ",Ê
Gentamicin (see dosing section, p. 147)
UÊÊ ÕÀ>Ì\ÊÇq£{Ê`>ÞÃ
Hospitalized > 48H
UÊÊ ivi«iÊ£Ê}Ê6Ê+n
OR
UÊÊ* Ê>iÀ}Þ\ÊâÌÀi>Ê£Ê}Ê6Ê+nÊ",Ê
Gentamicin (see dosing section, p. 147)
UÊ ÕÀ>Ì\ÊÇq£{Ê`>ÞÃ
UÊÊ ivi«iÊ£Ê}Ê6Ê+n
OR
UÊÊ* Ê>iÀ}Þ\ÊâÌÀi>Ê£Ê}Ê6Ê+nÊ´Ê
Gentamicin (see dosing section, p. 147)
UÊ ÕÀ>Ì\ÊÇq£äÊ`>ÞÃ
6.17 Urinary tract infections
UÊÊ"À>Ê «ÀyÝ>VÊÀÊ/*É-8Ê >ÛiÊiÝViiÌÊ
bioavailability and should be used as step-down therapy
if organism is susceptible
UÊÊ"À>ÊLiÌ>>VÌ>ÃÊÃ Õ`ÊÌÊLiÊÕÃi`ÊvÀÊL>VÌiÀi>Ê
due to inadequate blood concentrations
UÊÊ ÕÀ>ÌÊvÊi«ÀVÊ6ÊÌ iÀ>«ÞÊÃ Õ`ÊLiÊVÕÌi`Ê
towards total duration of therapy
Notes
UÊÊ"À>ÊÃÌi«`ÜÊÌ iÀ>«ÞÊÃ Õ`ÊLiÊÕÃi`ÊvÊÀ}>ÃÊÃÊ
susceptible
UÊÊ ÕÀ>ÌÊvÊi«ÀVÊ6ÊÌ iÀ>«ÞÊÃ Õ`ÊLiÊVÕÌi`Ê
towards total duration of therapy
"À>ÊÃÌi«`ÜÊÌ iÀ>«ÞÊvÊÀ}>ÃÊÃÊÃÕÃVi«ÌLi\
UÊ «ÀyÝ>VÊxääÊ}Ê*"Ê+£ÓÊvÀÊÇÊ`>ÞÃÊ
UÊ/*É-8Ê£Ê -Ê*"Ê+£ÓÊvÀÊÇ£äÊ`>ÞÃÊ
UÊ iv«`ÝiÊ{ääÊ}Ê*"Ê+£ÓÊvÀÊ£{Ê`>ÞÃÊ
UÊÊ"À>ÊÃvÞVÊV>ÊLiÊVÃ`iÀi`ÊvÊÃÕÃVi«ÌLiÊvÀÊ
Gram-negative MDR organisms (susceptibilities must be
requested). Consult ID Pharmacist for dosing.
6.17 Urinary tract infections
DIAGNOSIS
Specimen collection\Ê/ iÊÕÀiÌ À>Ê>Ài>ÊÃ Õ`ÊLiÊVi>i`ÊÜÌ Ê>Ê
antiseptic cloth and the urine sample should be collected midstream
or obtained by fresh catheterization. Specimens collected using
a drainage bag or taken from a collection hat are not reliable and
should not be sent.
Interpretation of the urinalysis (U/A) and urine culture
UÊÊ1À>ÞÃÃÊ>`ÊÕÀiÊVÕÌÕÀiÃÊÕÃÌÊLiÊÌiÀ«ÀiÌi`ÊÌ}iÌ iÀÊÊ
context of symptoms
UÊUrinalysis/microscopy:
UÊÊ «ÃÌV
UÊ ÌÀÌiÃÊ`V>ÌiÊL>VÌiÀ>ÊÊÌ iÊÕÀi
UÊiÕVÞÌiÊiÃÌiÀ>ÃiÊ`V>ÌiÃÊÜ ÌiÊL`ÊViÃÊÊÌ iÊÕÀi
UÊÊ >VÌiÀ>\Ê«ÀiÃiViÊvÊL>VÌiÀ>ÊÊÕÀ>ÞÃÃÊÃ Õ`ÊLiÊ
interpreted with caution and is not generally useful
UÊÊ*ÞÕÀ>Ê­ÀiÊÃiÃÌÛiÊÌ >ÊiÕVÞÌiÊiÃÌiÀ>Ãi®\Ê£äÊ7 É «vÊÀÊ
>27 WBC/microliter
UÊ1ÀiÊVÕÌÕÀiÃ\
UÊÊvÊ1ÉÊÃÊi}>ÌÛiÊvÀÊ«ÞÕÀ>]Ê«ÃÌÛiÊVÕÌÕÀiÃÊ>ÀiÊiÞÊ
contamination
UÊÊÃÌÊ«>ÌiÌÃÊÜÌ Ê1/ÊÜÊ >ÛiÊ100,000 colonies of a
uropathogen. Situations in which lower colony counts may be
Ã}wV>ÌÊVÕ`i\Ê«>ÌiÌÃÊÜ Ê>ÀiÊ>Ài>`ÞÊÊ>ÌLÌVÃÊ>ÌÊÌ iÊ
time of culture, symptomatic young women, suprapubic aspiration,
and men with pyuria.
TREATMENT NOTES
UÊÊ*ÞÕÀ>ÊiÌ iÀÊÊÌ iÊÃiÌÌ}ÊvÊi}>ÌÛiÊÕÀiÊVÕÌÕÀiÃÊÀÊÊ«>ÌiÌÃÊ
with asymptomatic bacteriuria usually requires no treatment. If
pyuria persists consider other causes (e.g. interstitial nephritis or
cystitis, fastidious organisms).
UÊÊÜÕ«ÊÕÀiÊVÕÌÕÀiÃÊÀÊ1ÉÊ>ÀiÊÞÊÜ>ÀÀ>Ìi`ÊvÀÊ}}Ê
symptoms. They should NOT be acquired routinely to monitor
response to therapy.
UÊÊ-iiÊ«°Ê££{ÊvÀÊ`ÃVÕÃÃÊvÊÌÀi>ÌiÌÊ«ÌÃÊvÀÊ6, Ê>`ÊÀi>Ê
concentrations of antibiotics.
112
Category
Asymptomatic
bacteriuria
Definition
Positive urine culture
100,000 CFU/mL
with no signs or
symptoms of infection
Empiric treatment
Remove the catheter
ÊÌÀi>ÌiÌÊÕiÃÃÊÌ iÊ«>ÌiÌÊÃ\
UÊ*Ài}>ÌÊ
UÊLÕÌÊÌÊÕ`iÀ}Ê>ÊÕÀ}VÊ«ÀVi`ÕÀiÊ
UÊ*ÃÌÊÀi>ÊÌÀ>ë>Ì
"/ \ÊLÌ>}Ê
UÊ iÕÌÀ«iV
routine cultures in
Antibiotics do not decrease asymptomatic
asymptomatic patients bacteriuria or prevent subsequent development
is not recommended
of UTI
Signs and symptoms
CatheterUÊÊ,iÛiÊV>Ì iÌiÀÊÜ iÊ«ÃÃLi
associated UTI (fever with no other
Patient stable with no evidence of upper tract
source is the most
(CA-UTI)
`Ãi>Ãi\
VÆÊ«>ÌiÌÃÊ>ÞÊ UÊÊvÊV>Ì iÌiÀÊÀiÛi`]ÊVÃ`iÀÊLÃiÀÛ>ÌÊ>i
also have suprapubic
OR
or flank pain)
UÊÊ ÀÌ>«iiÊ£Ê}Ê6Ê+Ó{
AND pyuria (10
OR
WBC/hpf)
UÊÊ ivÌÀ>ÝiÊ£Ê}Ê6Ê+Ó{
AND positive urine
OR
culture 1,000
UÊÊ «ÀyÝ>VÊxääÊ}Ê*"Ê ÊÀÊ{ääÊ}Ê6Ê+£ÓÊ
CFU/mL (see
(avoid in pregnancy and in patients with prior
information below
exposure to quinolones)
regarding significant
UÊ ÕÀ>Ì\ÊÃiiÊLiÜ
colony counts)
Patient severely ill, with evidence of upper tract
disease, or hospitalized {nÊ\
UÊÊ ivi«iÊ£Ê}Ê6Ê+nÊ
OR
UÊ* Ê>iÀ}Þ\ÊâÌÀi>Ê£Ê}Ê6Ê+n
UÊ ÕÀ>Ì\ÊÃiiÊLiÜ
Urosepsis in a SIRS with urinary
UÊ*«iÀ>VÉÌ>âL>VÌ>ÊΰÎÇxÊ}Ê6Ê+È
source and
patient with
If prior urine culture data are available, tailor
nephrostomy tubes
nephrostomy
therapy based on those results
tubes
DIAGNOSIS
-«iViÊViVÌ\ The urine sample should be drawn from the
catheter port using aseptic technique, NOT from the urine collection
bag. In patients with long term catheters ( 2 weeks), replace the
catheter before collecting a specimen. Urine should be collected before
antibiotics are started.
-Þ«ÌÃ\ Catheterized patients usually lack typical UTI symptoms.
-Þ«ÌÃÊV«>ÌLiÊÜÌ Ê 1/ÊVÕ`i\
UÊÊ iÜÊviÛiÀÊÀÊÀ}ÀÃÊÜÌ ÊÊÌ iÀÊÃÕÀVi
UÊÊ iÜÊÃiÌÊ`iÀÕ]Ê>>Ãi]ÊiÌ >À}ÞÊÜÌ ÊÊÌ iÀÊÃÕÀVi
UÊÊ 6ÊÌi`iÀiÃÃ]Êy>Ê«>]Ê«iÛVÊ`ÃVvÀÌ
UÊÊVÕÌiÊ i>ÌÕÀ>
Interpretation of the urinalysis (U/A) and urine culture
UÊÊ*ÞÕÀ>\ÊÊÌ iÊ«ÀiÃiViÊvÊ>ÊV>Ì iÌiÀ]Ê«ÞÕÀ>Ê`iÃÊÌÊVÀÀi>ÌiÊÜÌ Ê
the presence of symptomatic CA-UTI and must be interpreted based
on the clinical scenario. The absence of pyuria suggests an alternative
diagnosis.
UÊÊ*ÃÌÛiÊÕÀiÊVÕÌÕÀi\Ê 1,000 colonies
113
6.17 Urinary tract infections
Management of patients WITH a urinary catheter
6.17 Urinary tract infections
DURATION
The duration of treatment has not been well studied for CA-UTI and
optimal duration is not known.
UÊÊÇÊ`>ÞÃÊvÊ«À«ÌÊÀiÃÕÌÊvÊÃÞ«ÌÃ
UÊÊ£äq£{Ê`>ÞÃÊvÊ`i>Þi`ÊÀiëÃi
UÊÊÎÊ`>ÞÃÊvÊV>Ì iÌiÀÊÀiÛi`ÊÊvi>iÊ«>ÌiÌÊ 65 years with lower
tract infection.
TREATMENT NOTES
UÊÊ,iÛiÊÌ iÊV>Ì iÌiÀÊÜ iiÛiÀÊ«ÃÃLi
UÊÊ,i«>ViÊV>Ì iÌiÀÃÊÌ >ÌÊ >ÛiÊLiiÊÊ 2 weeks if still indicated
UÊÊ*À« Þ>VÌVÊ>ÌLÌVÃÊ>ÌÊÌ iÊÌiÊvÊV>Ì iÌiÀÊÀiÛ>ÊÀÊÀi«>ViiÌÊ
are NOT recommended due to low incidence of complications and
concern for development of resistance.
UÊÊ >Ì iÌiÀÊÀÀ}>ÌÊÃ Õ`ÊÌÊLiÊÕÃi`ÊÀÕÌiÞ
Treatment of Enterococci
UÊÊÊÃÌÊ>ÊE. faecalis isolates are susceptible to Amoxicillin 500 mg
PO TID OR Ampicillin 1 g IV Q6H and should be treated with these
>}iÌðÊÀÊ«>ÌiÌÃÊÜÌ Ê* Ê>iÀ}Þ\Ê ÌÀvÕÀ>ÌÊ­Ê>VÀL`®)
£ääÊ}Ê*"Ê+£ÓÊ­`Ê "/ÊÕÃiÊÊ«>ÌiÌÃÊÜÌ Ê À ÊÊxäÊÉ®°Ê
UÊE. faecium (often Vancomycin resistant)
UÊÊ ÌÀvÕÀ>ÌÊ­>VÀL`®) 100 mg PO Q12H if susceptible (do NOT
use in patients with CrCl 50 mL/min).
UÊ/iÌÀ>VÞViÊxääÊ}Ê*"Ê+ÈÊvÊÃÕÃVi«ÌLi
UÊÊÃvÞVÊÎÊ}Ê*"ÊViÊ­vÊvi>iÊÜÌ ÕÌÊV>Ì iÌiÀÊÀÊV>Ì iÌiÀÊ
ÃÊÀiÛi`ÆÊ>ÃÊÌ iÊVÀÊ>LÊvÀÊÃÕÃVi«ÌLÌÞ®
UÊÊiâ`ÊÈääÊ}Ê*"Ê Ê",ÊÃvÞVÊÎÊ}Ê*"ÊiÛiÀÞÊÓqÎÊ`>ÞÃÊ
(max 21 days) if complicated UTI or catheter can not be removed
Renal excretion/concentration of selected antibiotics
Good (≥60%): aminoglycosides, Amoxicillin, Amoxicillin/clavulanate,
Fosfomycin, Cefazolin, Cefepime, Cephelexin, Ciprofloxacin,
Colistin, Ertapenem, Trimethoprim/sulfamethoxazole, Vancomycin,
Amphotericin B, Fluconazole, Flucytosine
Variable (30-60%):Ê iv«`Ýi]Êiâ`Ê­Î䯮]Ê ÝÞVÞViÊ
­Óqxx¯®]Ê ivÌÀ>Ýi]Ê/iÌÀ>VÞViÊ­HÈ䯮ÊÊ
Poor (<30%): Azithromycin, Clindamycin, Moxifloxacin, Oxacillin,
Tigecycline, Micafungin, Posaconazole, Voriconazole
,iviÀiViÃ\
*ÞÕÀ>Ê>`ÊÕÀ>ÀÞÊV>Ì iÌiÀÃ\ÊÀV ÊÌÊi`ÊÓäääÆ£Èä­x®\ÈÇÎÇÇ°
IDSA Guidelines for treatment of uncomplicated acute bacterial cystitis and
«Þii« ÀÌÃÊÊÜi\Ê ÊviVÌÊ ÃÊ£ÆÓ\Ç{x°
-ÊÕ`iiÃÊvÀÊÌÀi>ÌiÌÊvÊ 1/\Ê ÊviVÌÊ ÃÊÓä£äÆxä\ÈÓxqÈΰ
114
Oropharyngeal disease (thrush)
Initial treatment
UÊÊ ÌÀ>âiÊ£äÊ}ÊÌÀV iÊxÊÌiÃÊ>Ê`>Þ
OR
UÊ ÞÃÌ>ÌÊÃÕëiÃÊxää]äääÊÕÌÃÉxÊ{ÊÌiÃÊ>Ê`>Þ
Recurrent or intractable disease
UÊÕV>âiÊ£ääqÓääÊ}Ê*"ÊViÊ`>Þ
Duration: xq£äÊ`>ÞÃ
NOTE: If refractory to Fluconazole consider fungal culture and
susceptibilities
Esophageal candidiasis
Initial treatment
UÊÕV>âiÊÓääq{ääÊ}Ê6É*"ÊViÊ`>Þ
Duration: £{qÓ£Ê`>ÞÃ
Relapse
UÊÊÕV>âiÊ{ääqnääÊ}Ê6É*"ÊViÊ`>Þ
Refractory to Fluconazole 800 mg daily (fungal culture and
susceptibilities are recommended)
UÊV>vÕ}Ê£xäÊ}Ê6ÊViÊ`>Þ
OR
UÊ« ÌiÀVÊ Êä°Îqä°ÇÊ}É}Ê6ÊViÊ`>Þ
OR
UÊ"À>ÊÌ iÀ>«Þ\ÊÌÀ>V>âiÊÀ>ÊÃÕÌÊÓääÊ}Ê`>Þ
Duration: £{qÓ£Ê`>ÞÃ
Candiduria
UÊ1À>ÀÞÊV>Ì iÌiÀÊÀiÛ>ÊÜÊÀiÃÛiÊÌ iÊV>``ÕÀ>ÊÊ{ä¯ÊvÊV>Ãið
TREATMENT
Asymptomatic cystitis
UÊ/ iÀ>«ÞÊÌÊÕÃÕ>ÞÊ`V>Ìi`
UÊÊ Ã`iÀÊÊÌ iÊvÜ}ÊV`ÌÃÊ­ÃiiÊÀi}iÃÊÕ`iÀÊ
ºÃÞ«Ì>ÌVÊVÞÃÌÌû®\
UÊ iÕÌÀ«iVÊ«>ÌiÌÃÊ
UÊ,i>ÊÌÀ>ë>Ì
UÊ1À>ÀÞÊLÃÌÀÕVÌÊÀÊ>LÀ>Ê1ÊÌÀ>VÌ
UÊ7 iÊÀiVÛiÀi`ÊÊÕÀiÊ«ÀÀÊÌÊÕÀ}VÊ«ÀVi`ÕÀiÃ
115
6.18 Candidiasis in the non-neutropenic patient
Candidiasis in the non-neutropenic patient
6.18 Candidiasis in the non-neutropenic patient
Symptomatic cystitis
Preferred therapy
UÊÊÕV>âiÊÓääÊ}Ê6É*"ÊViÊ`>ÞÊ
Duration:ÊÇq£{Ê`>ÞÃ
Fluconazole-resistant organism suspected or confirmed
UÊ« ÌiÀVÊ Êä°Îä°ÈÊ}É}Ê6ÊViÊ`>ÞÊ
Duration:Ê£qÇÊ`>ÞÃÊ
Pyelonephritis
NOTE: Candida pyelonephritis is usually secondary to hematogenous
spread except for patients with renal transplant or abnormalities of the
urogenital tract.
Preferred therapy
UÊÕV>âiÊÓääq{ääÊ}Ê6É*"ÊViÊ`>ÞÊ
Duration: 14 days
Fluconazole-resistant organism suspected or confirmed
UÊ« ÌiÀVÊ Êä°xqä°ÇÊ}É}Ê6ÊViÊ`>ÞÊ
OR
UÊV>vÕ}Ê£ääÊ}Ê6ÊViÊ`>ÞÊ
Duration: 14 days
TREATMENT NOTES
UÊ,iÛiÊÕÀ>ÀÞÊV>Ì iÌiÀÊvÊ«ÃÃLi°
UÊÊ/ iÀ>«ÞÊvÊV>``ÕÀ>ÊÊÌ iÊiÕÌÀ«iV]Ê 1ÊV>Ì iÌiÀâi`Ê
patient has not been shown to be beneficial and promotes resistance.
UÊÊ Ãi®, Voriconazole, Itraconazole, and Posaconazole are not
recommended due to poor penetration into the urinary tract.
UÊÊV>vÕ}Ê«iiÌÀ>ÌiÃÊ«ÀÞÊÊÌ iÊÕÀi]ÊLÕÌÊ`iÃÊ«iiÌÀ>ÌiÊÌÊ
renal tissue.
UÊ« ÌiÀVÊ ÊL>``iÀÊÜ>Ã iÃÊ>ÀiÊÌÊÀiVi`i`°
Candida vaginitis
Initial Therapy
UÊÕV>âiÊ£xäÊ}Ê*"Ê8Ê£Ê`ÃiÊ
OR
UÊV>âiÊÓ¯ÊVÀi>ÊxÊ}ÊÌÀ>Û>}>ÞÊViÊ`>ÞÊ8ÊÇÊ`>ÞÃ
Recurrent (> 4 episodes/year of symptomatic infection)
UÊÊÕV>âiÊ£xäÊ}Ê*"Ê+ÇÓÊ8ÊÎÊ`ÃiÃ]ÊÌ iÊ£xäÊ}Ê>ÊÜiiÊ8Ê
6 months
116
UÊÊ9 -/Ê ÊÊ "" Ê 1/1, Ê-"1 Ê "/Ê Ê " - , ÊÊ
CONTAMINANT.
NOTE: Micafungin does not have activity against Cryptococcus
TREATMENT
Unspeciated candidemia
Patients who are clinically stable and have not received prior long-term
azole therapy
UÊÕV>âiÊnääÊ}Ê6É*"Ê8Ê£Ê`Ãi]ÊÌ iÊ{ääÊ}Ê6É*"ÊViÊ`>Þ
Patients who are NOT clinically stable due to Candidemia or have
received prior long-term azole therapy
UÊV>vÕ}Ê£ääÊ}Ê6ÊViÊ`>ÞÊ
If the yeast is C. albicans or C. glabrata based on PNA FISH results,
follow the recommendations for C. albicans or C. glabrata noted below.
Otherwise, await speciation before modifying therapy as recommended
below, unless the patient becomes clinically unstable on Fluconazole.
Candida albicans
UÊÕV>âiÊnääÊ}Ê6É*"Ê8Ê£Ê`Ãi]ÊÌ iÊ{ääÊ}Ê6É*"ÊViÊ`>Þ
Patients who are NOT clinically stable due to Candidemia or have
received prior long-term azole therapy
UÊV>vÕ}Ê£ääÊ}Ê6ÊViÊ`>ÞÊ
Patients should be transitioned to Fluconazole once stable.
Candida glabrata
UÊV>vÕ}Ê£ääÊ}Ê6ÊViÊ`>Þ
OR
UÊÊÕV>âiÊnääÊ}Ê6É*"Ê8Ê£Ê`Ãi]ÊÌ iÊ{ääÊ}Ê6É*"ÊViÊ`>ÞÊÊ
the isolate is susceptible with MIC 8 mcg/mL and the patient is stable.
If isolate is intermediate to Fluconazole and oral therapy is desired,
consult ID. Other azoles such as Voriconazole should not be used in
Fluconazole-resistant strains due to the same mechanism of resistance.
Candida krusei
UÊV>vÕ}Ê£ääÊ}Ê6ÊViÊ`>ÞÊ
Fluconazole should NEVER be used to treat infections due to C. krusei
because the organism has intrinsic resistance to Fluconazole. This
iV >ÃÊvÊÀiÃÃÌ>ViÊÃÊÌÊÃ >Ài`ÊÜÌ Ê6ÀV>âiÆÊÌ iÀivÀi]Ê
oral Voriconazole can be used if isolate is susceptible (for dosing see
Voriconazole specific guidelines, p. 19).
117
6.18 Candidiasis in the non-neutropenic patient
Candidemia
6.18 Candidiasis in the non-neutropenic patient
Candida lusitaniae
UÊÕV>âiÊnääÊ}Ê6É*"Ê8Ê£Ê`Ãi]ÊÌ iÊ{ääÊ}Ê6É*"ÊViÊ`>Þ
C. lusitaniaeÊÃÊÀiÃÃÌ>ÌÊÌÊ« ÌiÀVÊ ÊÊ>««ÀÝ>ÌiÞÊÓä¯ÊvÊ
cases.
Candida parapsilosis
UÊÕV>âiÊnääÊ}Ê6É*"Ê8Ê£Ê`Ãi]ÊÌ iÊ{ääÊ}Ê6É*"ÊViÊ`>Þ
Fluconazole-intermediate isolate
UÊÕV>âiÊnääÊ}Ê6É*"ÊViÊ`>Þ
Fluconazole-resistant isolate
UÊV>vÕ}Ê£ääÊ}Ê6ÊViÊ`>Þ
If the patient is not responding to Micafungin then consider changing
to Amphotericin B. The minimum inhibitory concentrations (MICs) of
echinocandins are higher for C. parapsilosis than any other Candida
spp.ÆÊÌ ÃÊ >ÃÊi`ÊÌÊVViÀÊÌ >ÌÊÃiÊviVÌÃÊÜÌ ÊC. parapsilosis
may not respond well to echinocandins.
Candida tropicalis
UÊÕV>âiÊnääÊ}Ê6É*"Ê8Ê£Ê`Ãi]ÊÌ iÊ{ääÊ}Ê6É*"ÊViÊ`>Þ
Fluconazole-intermediate isolate
UÊÕV>âiÊnääÊ}Ê6É*"ÊViÊ`>Þ
Fluconazole-resistant isolate
UÊV>vÕ}Ê£ääÊ}Ê6ÊViÊ`>Þ
TREATMENT NOTES
Amphotericin B use in Candidemia
UÊÊ« ÌiÀVÊ ÊÃÊ } ÞÊivviVÌÛiÊ>}>ÃÌÊ>ÊCandida spp. except
for C. lusitaniaeÆÊ ÜiÛiÀ]Ê>âiÃÊ>`ÊiV V>`ÃÊ>ÀiÊv>ÛÀi`ÊÊ
susceptible strains over Amphotericin B products due to toxicity.
Doses for Candidemia
UÊ« ÌiÀVÊ Êä°ÇÊ}É}Ê6ÊViÊ`>Þ
OR
UÊÊ Ãi® 3 mg/kg IV once daily (if patient cannot tolerate
conventional Amphotericin B)
Duration
UÊÊ£{Ê`>ÞÃÊvÜ}Ê`VÕiÌi`ÊVi>À>ViÊvÊL`ÊVÕÌÕÀiÃÊ>`ÊVV>Ê
symptoms
UÊÊ*>ÌiÌÃÊÜÌ Ê«iÀÃÃÌiÌÊV>``i>Ê>`ÉÀÊiÌ>ÃÌ>ÌVÊV«V>ÌÃÊ
(e.g. endophthalmitis, endocarditis) need a longer duration of therapy
and evaluation by Ophthalmology and ID.
118
6.18 Candidiasis in the non-neutropenic patient
Ê
Hidden Content
- JHH Internal use only
Non-pharmacologic management
UÊÊ,iÛ>ÊvÊ>ÊiÝÃÌ}ÊViÌÀ>ÊÛiÕÃÊV>Ì iÌiÀÃÊÃÊ } ÞÊ
recommended.
UÊÊ*>ÌiÌÃÊÃ Õ`Ê >ÛiÊL`ÊVÕÌÕÀiÃÊ`>ÞÊÀÊiÛiÀÞÊÌ iÀÊ`>ÞÊÕÌÊ
candidemia is cleared.
UÊÊ*>ÌiÌÃÊÃ Õ`Ê >ÛiÊ>Ê« Ì >}VÊiÝ>>ÌÊÌÊiÝVÕ`iÊ
candidal endophthalmitis prior to discharge, preferably once the
candidemia is controlled.
UÊÊ V V>À`}À>« ÞÊV>ÊLiÊVÃ`iÀi`ÊvÊÌ iÊ«>ÌiÌÊ >ÃÊ«iÀÃÃÌiÌÊ
candidemia on appropriate therapy.
Endophthalmitis
UÊ>>}iiÌÊÊVÕVÌÊÜÌ Ê"« Ì >}Þ
UÊÊ ÕiÊÌÊ«ÀÊ -Ê>`ÊÛÌÀi>Ê«iiÌÀ>Ì]ÊÌÀi>ÌiÌÊÜÌ ÊiV V>`ÃÊ
is NOT recommended.
Preferred therapy
UÊ« ÌiÀVÊ Ê£Ê}É}Ê6ÊViÊ`>ÞÊ´ÊÕVÞÌÃiÊÓxÊ}É}Ê*"Ê+È
OR
UÊ Ãi®ÊxÊ}É}Ê6ÊViÊ`>ÞÊ´ÊÕVÞÌÃiÊÓxÊ}É}Ê*"Ê+È
Alternate therapy
UÊÊÕV>âiÊ{äänääÊ}Ê6É*"ÊViÊ`>ÞÊ´ÊÕVÞÌÃiÊÓxÊ}É}Ê
PO Q6H
Duration: {qÈÊÜiiÃ
Endocarditis
Consultation with ID and Cardiac Surgery is recommended. Surgical
valve replacement is considered a critical component for cure. If
the patient is not a candidate for surgery then life-long Fluconazole
suppression is likely required.
119
6.18 Candidiasis in the non-neutropenic patient
Preferred therapy
UÊ ÃiÁÊxÊ}É}Ê6ÊViÊ`>Þ
Alternative therapy
UÊÊV>vÕ}Ê£xäÊ}Ê6ÊViÊ`>ÞÊ´ÊÕV>âiÊ{ääqnääÊ}Ê6É*"Ê
once daily
Duration: 6 weeks or longer
Notes on antifungal susceptibility testing
UÊÊ-ÕÃVi«ÌLÌÞÊÌiÃÌ}ÊvÀÊÕV>âi]ÊÌÀ>V>âi]Ê6ÀV>âi]Ê
Flucytosine, and Micafungin is performed routinely on the first yeast
isolate recovered from blood.
UÊÊÕV>âiÊ>`ÊV>vÕ}ÊÃÕÃVi«ÌLÌÞÊ>ÀiÊÀi«ÀÌi`ÊÊ>ÊÃ>Ìið
UÊÊ"À}>ÃÃÊÌ >ÌÊ >ÛiÊV>vÕ}Ê ÃÊÊÌ iÊÀ>}iÊvÊ£qÓÊV}ÉÊ
(reported as susceptible) may not respond to treatment. ID consult is
recommended in these cases.
UÊÊ-ÕÃVi«ÌLÌÞÊÌiÃÌ}ÊvÀÊVÛiÌ>Ê« ÌiÀVÊ ÊÃÊ`iÊÀÕÌiÞÊ
for C. lusitaniae and C. guillermondii, and for other organisms by
request.
UÊÊvÊÌ iÊÀ}>ÃÊÃÊÌiÀi`>ÌiÊ­®ÊÌÊÕV>âi]ÊÌ iÊnääÊ}Ê6É
PO once daily can be used. This choice is NOT recommended in an
immunocompromised patient, in a patient who is clinically unstable
due to candidemia, or in patients with endocarditis, meningitis or
endophthalmitis.
UÊ-ÕÃVi«ÌLÌÞÊÌiÃÌ}ÊÃ Õ`ÊLiÊVÃ`iÀi`ÊÜ i\
UÊÕVVÕÌ>iÕÃÊV>``>ÃÃÊÃÊÀivÀ>VÌÀÞÊÌÊÕV>âi
UÊÊ/Ài>Ì}ÊÃÌiÞiÌÃ]Êi}ÌÃ]ÊÀÊi`« Ì >ÌÃÊÜÌ Ê
Fluconazole
UÊ `ÊVÕÌÕÀiÃÊ>ÀiÊ«iÀÃÃÌiÌÞÊ«ÃÌÛiÊÊÕV>âi
UÊÊ ÀÕÌiÊÃÕÃVi«ÌLÌÞÊÌiÃÌ}ÊV>ÊLiÊ>ÀÀ>}i`ÊLÞÊV>}ÊÌ iÊ
mycology lab at 5-6148
Notes on Fluconazole prophylaxis
UÊÊÕV>âiÊ«À« Þ>ÝÃÊà Õ`ÊLiÊÌi`ÊÌÊÌ iÊvÜ}ÊÃiÌÌ}Ã
UÊÊ*>ÌiÌÃÊiÝ«iVÌi`ÊÌÊÀi>ÊÊÌ iÊSICU or WICU for ≥ 72 hours
­ ÀÌiÀ>ÊvÀÊ«ÃÊ- 1Ê«À« Þ>ÝÃÊÃÌÕ`ÞÆÊ«À« Þ>ÝÃÊÊÌ iÀÊ
ICUs has NOT been studied and is NOT recommended).
UÊÊ iÕÌÀ«iVÊ«>ÌiÌÃÊÕ`iÀ}}ÊLiÊ>ÀÀÜÊÌÀ>ë>Ì>ÌÊÀÊ
treatment for leukemia/lymphoma
UÊÊ*>ÌiÌÃÊÜ Ê>ÀiÊ«ÃÌ«ÊvÀÊÛiÀÊÀÊ«>VÀi>ÃÊÌÀ>ë>Ìð
UÊÊÕV>âiÊ«À« Þ>ÝÃÊà Õ`ÊLiÊÃÌ««i`ÊÜ iÊ- 1ÊÀÊ7 1Ê
patients are transferred to the floor
,iviÀiViÃ\
-ÊÕ`iiÃÊvÀÊ/Ài>ÌiÌÊvÊ >``>ÃÃ\Ê ÊviVÌÊ ÃÊÓääÆ{n\xäÎxÎx°
ÕV>âiÊ«À« Þ>ÝÃÊÊÃÕÀ}V>Ê«>ÌiÌÃ\ÊÊ-ÕÀ}ÊÓää£ÆÓÎÎ\x{Óqn°
120
ÀÊëiVwVÊ«ÀVi`ÕÀiÃÊ>`Ê>}iÌÃÊÃiiʺ*iÀ«iÀ>ÌÛiÊ>ÌLÌVÊ
«À« Þ>ÝÃÊ`VÕiÌ»Ê>ÌÊÜÜÜ°Ã`i «Ãi`Vi°À}É>«
Drug
iv>âÊ
ivÌiÌ>Ê
Clindamycin
Ciprofloxacin
Gentamicin
Metronidazole
6>VÞVÊ
Ê
Ê
Usual dose
Ê£ÓäÊ}\ÊÓÊ}Ê
≥Ê£ÓäÊ}\ÊÎÊ}Ê
Ê£ÓäÊ}\ÊÓÊ}Ê
≥Ê£ÓäÊ}\ÊÎÊ}
600 mg
400 mg
5 mg/kg
500 mg
ÊÇäÊ}\Ê£Ê}Ê
Ç£Ê}\Ê£°ÓxÊ}
Ê£ääÊ}\Ê£°xÊ}
Redosing during procedure
+{Ê­+ÓÊvÀÊV>À`>VÊÃÕÀ}iÀÞ®
+{Ê­+ÓÊvÀÊV>À`>VÊÃÕÀ}iÀÞ®
+È
Q6H
None
None
None
+£Ó
Important notes
UÊÊ/}ÊÃÊVÀÕV>°ÊÌLÌVÃÊÕÃÌÊLiÊÊÌ iÊÃÊÜ iÊÌ iÊ
incision is made to be effective.
UÊÊ i« >ëÀÃÊV>ÊLiÊ>`ÃÌiÀi`ÊÛiÀÊÎqxÊÊ6Ê«Õà ÊÕÃÌÊLivÀiÊ
the procedure and will achieve appropriate skin levels in minutes.
Vancomycin and Ciprofloxacin must be given over 60 min. Clindamycin
à Õ`ÊLiÊvÕÃi`ÊÛiÀÊ£äqÓäÊ°Ê
UÊÊÀÊ>ÌLÌVÃÊÜÌ Ê}iÀÊvÕÃÊÌiÃÊ­i°}°Ê6>VÞV]Ê
Ciprofloxacin) the infusion should start 30 minutes prior to incision
UÊÊPost-procedure doses are NOT needed (exceptions are noted
in table). Single doses pre-procedure have been as effective as
post-procedure doses in all studies.
UÊÊ*>ÌiÌÃÊÀiViÛ}Ê«Ài«iÀ>ÌÛiÊ>ÌLÌVÃÊ}iiÀ>ÞÊ`Ê "/Êii`Ê
additional antibiotics for endocarditis prophylaxis.
UÊÊ*À« Þ>ÝÃÊvÀÊ«>ÌiÌÃÊ>Ài>`ÞÊÊ>ÌLÌVÃ\
UÊÊÀÊ>ÌLÌVÃÊÌ iÀÊÌ >Ê6>VÞV\Ê`ÊÃÌ>`}Ê`ÃiÊÕÌÊ
1 hour before incision
UÊÊÀÊ6>VÞV\Ê,i`ÃiÊ>ÊvÕÊ`ÃiÊvÊnÊ ÕÀÃÊ >ÛiÊ«>ÃÃi`ÊÃViÊ
the last dose or a half dose if fewer than 8 hours have passed in
patient with normal renal function
UÊÊiÌ>VÊÃ Õ`ÊLiÊ}ÛiÊ>ÃÊ>ÊÃ}iÊ`ÃiÊvÊxÊ}É}ÊÌÊ>ÝâiÊ
tissue penetration and minimize toxicity.
UÊÊvÊÊ`>ÞÃÃÊÀÊ À ÊÊÓäÊÉ]ÊÕÃiÊÓÊ}É}
UÊ ÊÌÊÀi`Ãi
UÊÊ1ÃiÊ>VÌÕ>ÊL`ÞÊÜi} ÌÊÕiÃÃÊ«>ÌiÌÊÃÊ≥ÊÓä¯ÊÛiÀÊ`i>ÊL`ÞÊ
weight (see p. 145)
121
6.19 Guidelines for use of prophylactic antimicrobials
Pre-operative and pre-procedure antibiotic
prophylaxis
6.19 Guidelines for use of prophylactic antimicrobials
Procedure
Urologic surgery/procedures
Transrectal prostate biopsy1
Transurethral surgery (e.g. TURP, TURBT,
ureteroscopy, cystouretoscopy)
Lithotripsy
Nephrectomy or radical prostatectomy
Radical cystectomy, ileal conduit,
cystoprostatectomy or anterior exenteration
*iiÊÀÊÌ iÀÊ«ÀÃÌ iÃiÃÊ
Cardiac surgery
Median sternotomy, heart transplant3
Median sternotomy, heart transplant with
previous VAD or MRSA colonization/infection3
Pacemaker or ICD insertion
Pacemaker or ICD insertion with MRSA
colonization/infection or generator exchange
VAD insertion
VAD insertion with MRSA colonization/infection
VAD insertion with open chest3
Lung transplant4
Prophylaxis
recommendations
PCN allergy
alternate prophylaxis
Cefazolin
Cefazolin
Ciprofloxacin OR Gentamicin2
Gentamicin2
Gentamicin2
Clindamycin
Clindamycin PLUS
Gentamicin2
Q iv>âÊ",Ê6>VÞVRÊÊQ `>ÞVÊ",Ê6>VÞVR
PLUS Gentamicin2
PLUS Gentamicin2
Cefazolin
Cefazolin
Cefotetan
Cefazolin
Cefazolin PLUS
Vancomycin
Cefazolin
Cefazolin PLUS
Vancomycin
Cefazolin
Cefazolin PLUS
Vancomycin
Cefazolin PLUS
Vancomycin
Cefepime
Vascular surgery
Carotid and brachiocephalic procedures
Prophylaxis not
without prosthetic grafts
recommended
Upper extremity procedures with prosthetic
Cefazolin
grafts and lower extremity procedures
L`>Ê>ÀÌ>Ê«ÀVi`ÕÀiÊÀÊ}ÀÊVÃÊÊ
ivÌiÌ>ÊÊ
Thoracic surgery
Lobectomy, pneumonectomy, lung resection, Cefazolin
thoracotomy, VATS
Esophageal cases
Cefotetan
Neurosurgery
Craniotomy, cerebrospinal fluid-shunting
procedures, implantation of intrathecal pumps
Laminectomy
Spinal fusion
Spinal fusion with MRSA colonization/infection
Vancomycin
Vancomycin
Clindamycin OR Vancomycin
Vancomycin
Vancomycin
Vancomycin
Vancomycin PLUS
Ciprofloxacin
Consult transplant ID
Prophylaxis not
recommended
Clindamycin OR Vancomycin
6>VÞVʳÊiÌ>V2
Clindamycin
Clindamycin
Cefazolin
Clindamycin
Clindamycin
Clindamycin OR Vancomycin
Vancomycin
Transsphenoidal procedures
Cefazolin
Cefazolin
Cefazolin PLUS
Vancomycin
Ceftriaxone
Orthopedic surgery
Clean operations involving hand, knee, or
foot, arthroscopy
Total joint replacement
Total joint replacement with MRSA
colonization/infection
Open reduction of fracture/internal fixation
Lower limb amputation
Prophylaxis not
recommended
Cefazolin
Cefazolin PLUS
Vancomycin
Cefazolin
Cefotetan
Prophylaxis not
recommended
Vancomycin
Vancomycin
Spinal fusion
Cefazolin
Spinal fusion with MRSA colonization/infection Cefazolin PLUS
Vancomycin
Laminectomy
Cefazolin
122
Moxifloxacin 400 mg
Clindamycin OR Vancomycin
Clindamycin PLUS
Gentamicin2
Clindamycin OR Vancomycin
Vancomycin
Clindamycin
Prophylaxis
recommendations
General surgery
*ÀVi`ÕÀiÃÊÛÛ}ÊiÌÀÞÊÌÊÕiÊvÊÕ««iÀÊÊ ivÌiÌ>Ê
GI tract, gastric bypass procedures,
pancreaticoduodenectomy, highly selective
vagotomy, Nissen fundoplication
>ÀÞÊÌÀ>VÌÊ«ÀVi`ÕÀiÃÊ­i°}°ÊV iVÞÃÌiVÌÞ]ÊÊ ivÌiÌ>Ê
choledochoenterostomy)
i«>ÌiVÌÞÊ
ivÌiÌ>Ê
Whipple procedure or pancreatectomy
Cefotetan
Small bowel procedures
Cefotetan
* Ê
Appendectomy (if complicated or perforated,
treat as secondary peritonitis)
Colorectal procedures, penetrating abdominal
trauma
Inguinal hernia repair
«V>Ìi`]ÊiiÀ}iÌÊÀÊÀi«i>ÌÊ}Õ>ÊÊ
hernia repair
Mastectomy
iv>âÊ",Ê ivÌiÌ>Ê
Cefotetan
Cefotetan
Cefazolin
ivÌiÌ>Ê
PCN allergy
alternate prophylaxis
`>ÞVÊ´ÊiÌ>V2
`>ÞVÊ´ÊiÌ>V2
`>ÞVÊ´ÊiÌ>V2
Clindamycin PLUS
Ciprofloxacin
Clindamycin PLUS
Gentamicin2
`>ÞVÊ´ÊiÌ>V2
Clindamycin PLUS
Gentamicin2
Clindamycin PLUS
Gentamicin2
Clindamycin
`>ÞVÊ´ÊiÌ>V2
Mastectomy with lymph node dissection
Prophylaxis not
recommended
Cefazolin
Prophylaxis not
recommended
Clindamycin PLUS
Gentamicin2
Gynecologic surgery
Cesarean delivery procedures
Cefazolin
Clindamycin PLUS
Gentamicin2
Clindamycin PLUS
Gentamicin2
Clindamycin PLUS
Gentamicin2
Clindamycin
Hysterectomy (vaginal or abdominal)
Cefazolin OR Cefotetan
Oncology procedures
Cefotetan
Repair of cystocele or rectocele
Cefazolin
Head and neck surgery
Parotidectomy, thyroidectomy, tonsillectomy
Prophylaxis not
recommended
Reconstructive procedure w/prosthesis
Cefazolin
placement
Adenoidectomy, rhinoplasty, tumor-debulking, Cefotetan OR Clindamycin
or mandibular fracture repair
Major neck dissection
Cefazolin
Plastic surgery
Clean with risk factors or clean-contaminated
Tissue expander insertion/implants/all flaps
Rhinoplasty
Prophylaxis not
recommended
Clindamycin
Clindamycin
Clindamycin
Cefazolin
Cefazolin
No prophylaxis OR
Cefazolin
Clindamycin
Clindamycin
No prophylaxis OR
Clindamycin
Abdominal transplant surgery
Pancreas or pancreas/kidney transplant
Cefotetan
Renal transplant/adult live donor
Liver transplant4
Cefazolin
Cefotetan
Clindamycin PLUS
Ciprofloxacin
Clindamycin
Clindamycin PLUS
Ciprofloxacin
1vÊ«Ài«ÊÀiVÌ>ÊÃVÀiiÊ«iÀvÀi`\ÊÃiiÊ«°Ê£Ó{Ê
2Do
not give additional doses of Gentamicin post-op for prophylaxis
open chest, continue antibiotic prophylaxis until closure
recommendations are for patients with no relevant microbiology data that would suggest
ÀiÃÃÌ>ÌÊÀ}>ÃÃÆÊ«À« Þ>VÌVÊÀi}iÊÃ Õ`ÊLiÊÌ>Ài`ÊL>Ãi`ÊÊÜÊVÀL}ÞÊ`>Ì>ÊÜÌ Ê
assistance of transplant ID (page in PING)
3For
4Listed
123
6.19 Guidelines for use of prophylactic antimicrobials
Procedure
6.19 Guidelines for use of prophylactic antimicrobials
Procedure
Prophylaxis
recommendations
PCN allergy
alternate prophylaxis
Interventional radiology procedures
>ÀÞÉÆÊV iÊiLâ>ÌÉÊÊ
ivÌiÌ>ÊÊ
`>ÞVÊ
percutaneous liver ablation (hx. of
PLUS Gentamicin
L>ÀÞÊÃÕÀ}iÀÞÉÃÌÀÕiÌ>Ì®ÆÊ
cecostomy
iÊiLâ>ÌÆÊwLÀ`ÉÕÀiÊ
*À« Þ>ÝÃÊÌÊ
>ÀÌiÀÞÊiLâ>ÌÆÊ«iÀVÕÌ>iÕÃÊÊ
ÀiVi`i`
ÛiÀÉÀi>ÉÕ}IÊ>L>ÌÆÊÛ>ÃVÕ>ÀÊ
vascular malformation embolization†
Urologic procedure (not ablation)
Cefazolin
Gentamicin
Lymphangiogram/embolization
Cefazolin
Clindamycin
Placement of tunneled catheters
Prophylaxis not
­i°}°ÊViÌÀ>Êi®ÆÊÛiÕÃÉ>ÀÌiÀ>ÊÊ
ÀiVi`i`
procedures.
Placement of implantable access
Cefazolin
Clindamycin
port (e.g. Mediport®)
*Pre-treatment w/ antibiotics can be considered for patients w/ COPD or h/o recurrent post-obstructive
pneumonia
† Lymphatic or patients w/ necrotic skin undergoing vascular graft should receive prophylaxis
w/Cefazolin
Prophylaxis for Prostate Biopsy Based on Rectal Screen Results
Pre-op prophylaxis regimen1
Post-op oral options2
Ciprofloxacin
susceptible
Ê
Ciprofloxacin 750 mg PO 2 hours
before procedure for any renal
vÕVÌÊÊÊ
Ciprofloxacin 500 mg PO once
12 hours after the procedure. If GFR
ÎäÊÉÊÊii`ÊvÀÊ«ÃÌ«Ê`Ãi°Ê
«ÀyÝ>VÊÊ
ÀiÃÃÌ>Ì]Ê/*É-8Ê
susceptible
/*É-8Ê£Ê -Ê£Ê ÕÀÊLivÀiÊÊ
«ÀVi`ÕÀi]Ê>`Ê£Ê -ÊÎÊ ÕÀÃÊÊ
before
/*É-8Ê£Ê -Ê*"ÊViÊ£ÓÊ ÕÀÃÊ
>vÌiÀÊÌ iÊ«ÀVi`ÕÀi°ÊvÊ,ÊÎäÊ
ml/min no need for post-op dose.
Ciprofloxacin and
/*É-8ÊÀiÃÃÌ>Ì]ÊÊ
Cefazolin susceptible
Cefazolin 2 g IV push (3-5 min)
ÜÌ Ê>Ê£Ê ÕÀÊvÊ«ÀVi`ÕÀiÊ
Cefpodoxime 100 mg PO once
OR
Cefdinir 300 mg PO once
Ciprofloxacin,
/*É-8]ÊÊ
Cefazolin resistant
Gentamicin 5 mg/kg IV once over
ÎäÈäÊÊÊ
OR
Ceftriaxone 1 g IV over 30 min if
susceptible
No need for additional doses as
iÌ>VÊ>`Ê ivÌÀ>ÝiÊÀiÌ>Ê
therapeutic levels for 24 hours
Other resistance
Call ID Pharmacist
patterns
1 All doses are for any renal function 2 Post-op antibiotics are not required by SCIP
124
NOTES:
UÊÊ*>ÌiÌÃÊÜ Ê >ÛiÊÀiViÛi`Ê>ÌLÌVÃÊvÀÊÃÕÀ}V>Ê«À« Þ>ÝÃÊ`ÊÌÊ
need additional prophylaxis for endocarditis.
Antibiotic prophylaxis solely to prevent endocarditis is not
recommended for GU or GI tract procedures.
Cardiac conditions associated with a high risk of endocarditis
for which prophylaxis is recommended prior to some dental and
respiratory tract procedures and procedures involving infected
skin or musculoskeletal tissue
UÊ*ÀÃÌ iÌVÊV>À`>VÊÛ>Ûi
UÊ*ÀiÛÕÃÊi«Ã`iÊvÊviVÌÛiÊi`V>À`ÌÃ
UÊ }iÌ>Ê i>ÀÌÊ`Ãi>ÃiÊ­ ®
UÊÊÊ1Ài«>Ài`ÊVÞ>ÌVÊ ]ÊVÕ`}Ê«>>ÌÛiÊÃ ÕÌÃÊ>`ÊV`ÕÌÃ
UÊÊ «iÌiÞÊÀi«>Ài`ÊV}iÌ>Ê i>ÀÌÊ`iviVÌÊÜÌ Ê«ÀÃÌ iÌVÊ
material or device, whether placed by surgery or by catheter
intervention, during the first 6 months after the procedure
UÊÊ,i«>Ài`Ê ÊÜÌ ÊÀiÃ`Õ>Ê`iviVÌÃÊ>ÌÊÌ iÊÃÌiÊÀÊ>`>ViÌÊÌÊÌ iÊ
site of a prosthetic patch or prosthetic device
UÊÊ >À`>VÊÌÀ>ë>Ì>ÌÊÀiV«iÌÃÊÜ Ê`iÛi«ÊV>À`>VÊÛ>ÛÕ«>Ì Þ
Antibiotic prophylaxis is recommended for the following dental
procedures ONLY:
UÊ>«Õ>ÌÊvÊ}}Û>ÊÌÃÃÕiÃÊÀÊ«iÀ>«V>ÊÀi}ÊvÊÌiiÌ
UÊ*iÀvÀ>ÌÊvÊÀ>ÊÕVÃ>
Antibiotic prophylaxis is recommended for the following
respiratory tract procedures ONLY:
UÊVÃÊÀÊL«ÃÞÊvÊÌ iÊÀiëÀ>ÌÀÞÊÕVÃ>
Antibiotic regimens
UÊÝVÊÓÊ}Ê*"Ê£Ê ÕÀÊLivÀiÊ«ÀVi`ÕÀi
OR
UÊ* Ê>iÀ}Þ\Ê `>ÞVÊÈääÊ}Ê*"Ê£Ê ÕÀÊLivÀiÊ«ÀVi`ÕÀi
OR
UÊ* Ê>iÀ}Þ\ÊâÌ ÀÞVÊxääÊ}Ê*"Ê£Ê ÕÀÊLivÀiÊ«ÀVi`ÕÀi
OR
UÊÊ*>ÌiÌÊÕ>LiÊÌÊÌ>iÊÀ>Êi`V>Ì\Ê«VÊÓÊ}ÊÉ6Ê£Ê ÕÀÊ
before procedure OR Cefazolin 1 g IM/IV 5 minute push prior to
procedure
,iviÀiVi\
ÊÕ`iiÃÊvÀÊ*ÀiÛiÌÊvÊviVÌÛiÊ `V>À`ÌÃ\Ê ÀVÕ>ÌÊÓääÇÆÊ££È\£ÇÎÈqx{°
125
6.19 Guidelines for use of prophylactic antimicrobials
Prophylaxis against bacterial endocarditis
6.19 Guidelines for use of prophylactic antimicrobials
Prophylactic antimicrobials for patients with
solid organ transplants
NOTE:ÊÊ`ÃiÃÊ>ÃÃÕiÊÀ>ÊÀi>ÊvÕVÌÆÊ`ÃiÊ`wV>ÌÃÊ>ÞÊLiÊ`V>Ìi`ÊvÀÊ
reduced CrCI.
Kidney, kidney-pancreas, pancreas transplants
Indication
Agent and dose
Duration
Anti-viral prophylaxis (CMV, HSV, VZV)
CMV D-/RAcyclovir 400 mg PO BID OR
Valacyclovir 500 mg PO BID
6Ê ³ÊÀÊ É,³Ê
6>}>VVÛÀ† 450 mg PO daily
6Ê ³É,Ê
6>}>VVÛÀ† 900 mg PO daily
3 months
3 months
6 months
Anti-fungal prophylaxis
Kidney
Clotrimazole troches 10 mg PO QID OR
Nystatin suspension 500,000 units QID
Pancreas and kidney
Fluconazole 400 mg PO daily
1 month‡
1 month
PCP prophylaxisÊ
Ê
Ê
ÀÃÌÊi\Ê/*É-8ÊiÊ--ÊÌ>LiÌÊ*"Ê`>ÞÊ
-iV`Êi\ÊÌÛ>µÕiÊ£xääÊ}Ê*"Ê`>Þ
/ À`Êi\Ê >«ÃiIÊ£ääÊ}Ê*"Ê`>ÞÊ",Ê
aerosolized Pentamidine
ÈÊÌ Ã
Acute rejection treated with Thymoglobulin or Muromonab (OKT3)
Anti-viral prophylaxis (CMV, HSV, VZV)
CMV D-/RAcyclovir 400 mg PO BID OR
3 months
Valacyclovir 500 mg PO BID
3 months
6Ê ³ÊÀÊ É,³Ê
6>}>VVÛÀ† 450 mg PO daily
3 months
6Ê ³É,Ê
6>}>VVÛÀ† 900 mg PO daily
Anti-fungal prophylaxis Clotrimazole troches 10 mg PO QID
1 month
PCP prophylaxis
Ê
Ê
ÀÃÌÊi\Ê/*É-8ÊiÊ--ÊÌ>LiÌÊ*"Ê`>ÞÊ
-iV`Êi\ÊÌÛ>µÕiÊ£xääÊ}Ê*"Ê`>Þ
/ À`Êi\Ê >«ÃiIÊ£ääÊ}Ê*"Ê`>ÞÊ",
aerosolized Pentamadine
ÈÊÌ Ã
Agent and dose
Duration
Liver transplants
Indication
Anti-viral prophylaxis (CMV, HSV, VZV)
CMV D-/RAcyclovir 400 mg PO BID OR
Valacyclovir 500 mg PO BID
6Ê ³ÊÀÊ É,³Ê
6>}>VVÛÀ† 450 mg PO daily
6Ê ³É,Ê
6>}>VVÛÀ† 900 mg PO daily,
followed by PCR monitoring
Anti-fungal prophylaxis Fluconazole 400 mg PO daily
PCP prophylaxisÊ
ÀÃÌÊi\Ê/*É-8ÊiÊ--ÊÌ>LiÌÊ*"Ê`>ÞÊÊ
Ê
ÌiÀ>ÌÛiÃ\ÊÌÛ>µÕiÊ£xääÊ}Ê*"Ê`>ÞÊ
or Dapsone 100 mg PO daily
126
3 months
3 months
6 months
6 weeks
£ÓÊÌ Ã
Indication
Agent and dose
Anti-viral prophylaxis (CMV, HSV, VZV)
6Ê É,Ê
Ê«À« Þ>ÝÃÊÕiÃÃÊ-6Ê}ÊÀÊ6<6Ê}ÊÊ
positive. If positive serology, Valacyclovir
500 mg PO BID
6Ê ³ÊÀÊ É,³Ê
6>}>VVÛÀ† 900 mg PO daily
6Ê ³É,Ê
6>}>VVÛÀ† 900 mg PO daily
Anti-fungal prophylaxis Nystatin suspension 500,000 units QID
PCP prophylaxisÊ
Ê
Ê
Ê
Duration
ÎÊÌ Ã
3 months
6 months
Until
prednisone
dose ≤ 10
mg/d x 3
months
ÀÃÌÊi\Ê/*É-8Ê--ÊiÊÌ>LiÌÊ*"Ê`>ÞÊ",Ê £ÓÊÌ Ã
Ê Ê /*É-8ÊiÊ -ÊÌ>LiÌÊ*"ÊÌ ÀiiÊÌiÃÉÜiiÊ
-iV`Êi\Ê >«ÃiIÊ£ääÊ}Ê*"Ê`>Þ
/ À`Êi\ÊÌÛ>µÕiÊ£xääÊ}Ê*"Ê`>ÞÊ
Toxoplasmosis prophylaxis
ÀÃÌÊi\Ê/*É-8ÊiÊ--ÊÌ>LiÌÊ*"Ê`>ÞÊÊ
£ÓÊÌ Ã
/ÝÊ,³Ê
Ê
-iV`Êi\Ê >«ÃiIÊ£ääÊ}Ê*"Ê`>ÞÊPLUS
Pyrimethamine and Leucovorin
/ÝÊ ³ÊÀÊÕÜÊ
ÀÃÌÊi\Ê/*É-8ÊiÊ--ÊÌ>LiÌÊ*"Ê`>ÞÊ
£ÓÊÌ ÃÊ
Ê Ê `ÀÊÃÌ>ÌÕÃÊ
-iV`Êi\Ê >«ÃiIÊ£ääÊ}Ê*"Ê`>ÞÊPLUS Lifelong
Pyrimethamine and Leucovorin
Lung transplants
Indication
Agent and dose
Duration
Anti-viral prophylaxis
CMV D-/RReceived
non-leukoreduced
or CMV unscreened
PRBCs
Ganciclovir 5 mg/kg IV Q12H x
14 days, then Ganciclovir 5 mg/kg IV
Q24H x 16 days, then Valacyclovir 500 mg
PO BID or Acyclovir 800 mg PO TID x 1 year
followed by Acyclovir 200 mg PO TID
Lifelong
CMV D-/RReceived leukoreduced
or CMV() PRBCs
Valacyclovir 500 mg PO BID or Acyclovir
Lifelong
800 mg PO TID x 1 year followed by Acyclovir
200 mg PO TID
6Ê ³ÊÀÊ É,³Ê
>VVÛÀÊxÊ}É}Ê6Ê+£ÓÊÝÊ£{Ê`>ÞÃ]ÊÌ iÊÊ vi}
Valganciclovir 900 mg PO daily x 3 months
(until CMV shell vial negative from 3 month
surveillance bronchoscopy), then Valacyclovir
500 mg po BID or Acyclovir 800 mg PO TID x
1 year, then Acyclovir 200 mg PO TID lifelong.
6Ê ³É,ÊÊÊ
>VVÛÀÊxÊ}É}Ê6Ê+£Ó ÊÝÊ£{Ê`>ÞÃ]ÊÌ iÊÊ vi}
Ganciclovir 5 mg/kg IV daily x 3 months, then
Valganciclovir 900 mg PO daily (until CMV shell
127
6.19 Guidelines for use of prophylactic antimicrobials
Heart transplants
6.19 Guidelines for use of prophylactic antimicrobials
vial negative from 6 month surveillance BAL),
then Valacyclovir 500 mg PO BID or Acyclovir
800 mg PO TID x 1 year, then Acyclovir 200 mg
PO TID lifelong.
Anti-fungal prophylaxis
No Aspergillus
Inhaled Amphotericin B per protocol
colonization
Ê
AspergillusÊVâ>ÌÊ
PCP prophylaxisÊ
Ê
Ê
Ê
ÞÃÌ>ÌÊxää]äääÊÕÌÃÊ Ê+ÈÊÕÌÊÊÊ
extubated, then Clotrimazole troches
10 mg PO Q6H until prednisone dose
10 mg daily
6ÀV>âiÊ­`Ãi`ÊLÞÊÜi} Ì®ÊÊÊ
ÊÈÊ}\Ê6ÀV>âiÊÓääÊ}Ê*"Ê 69 kg to Ê{Ê}\Ê6ÀV>âi
300 mg PO BID
Ê{Ê}\Ê6ÀV>âiÊ{ääÊ}Ê*"Ê ÀÃÌÊi\Ê/*É-8ÊiÊ -ÊÌ>LiÌÊ*"ÊÊ
Ê Ê Ì ÀiiÊÌiÃÉÜiiÊ",Ê/*É-8ÊiÊ
SS tablet PO daily
-iV`Êi\Ê >«ÃiIÊ£ääÊ}Ê*"Ê`>ÞÊÊ
/ À`Êi\ÊÌÛ>µÕiÊ£xääÊ}Ê*"Ê`>ÞÊ
During initial
hospitalization
stay
ÎqÈÊÌ ÃÊ
ÎqÈÊÌ ÃÊ
vi}
ÊrÊ`À]Ê,ÊrÊÀiV«iÌ]Ê­q®ÊrÊÃiÀi}>ÌÛi]Ê­³®ÊrÊÃiÀ«ÃÌÛi
NOTES:
/*É-8ÊÌ iÀ>«ÞÊÀi`ÕViÃÊÀÃÊvÊviVÌÊÜÌ ÊListeria spp., Nocardia spp., and
Toxoplasmosis, but does not eliminate risk.
For splenectomized patients, antibacterial prophylaxis with Amoxicillin 500 mg PO BID
(or Doxycycline if PCN allergy) is recommended for 1 year.
*Recommended screening for G6PD deficiency prior to initiation of Dapsone.
†If Valgancylovir is stopped prior to recommended duration of therapy due to intolerance,
recommend initiation of Acylovir or Valacyclovir for antiviral prophylaxis.
‡ /*qÎÊÌ Ã
128
NOTE: These guidelines were developed for use in BMT and leukemia
patients and may not be fully applicable in other instances.
Definitions
UÊ iÕÌÀ«i>\Ê ÊÊxääÉ3
UÊÊiÛiÀ\ÊÊ/i«ÊÊÎn°äcÊ ÊÌiÃÊÌÜÊ>ÌÊi>ÃÌÊÓÊ ÕÀÃÊ>«>ÀÌÊ",Ê
Temp > 38.3° C times one
TREATMENT
Always tailor antibiotics based on susceptibility profiles
vÊÌ iÊ«>ÌiÌÊÃÊ Þ«ÌiÃÛiÊÀÊÌ iÀÜÃiÊÕÃÌ>Li]ÊÃiiʺ/Ài>ÌiÌÊvÊ
VV>ÞÊÕÃÌ>LiÊ«>ÌiÌûʭ««ÃÌi®°
Initial fever
UÊÊ ivi«iÊÓÊ}Ê6Ê+nÊ´Ê6>VÞVIÊ­ÃiiÊ`Ã}ÊÃiVÌÊ«°Ê£xä®
OR
UÊ*«iÀ>VÉÌ>âL>VÌ>ÊΰÎÇxÊ}Ê6Ê+{Ê´Ê6>VÞVIÊ­ÃiiÊ`Ã}Ê
section p. 150)
I`V>ÌÃÊvÀÊ6>VÞV\ÊÃÕëiVÌi`Ê , -]ÊÃÊ>`ÊÃvÌÌÃÃÕiÊviVÌÃ]Ê
pneumonia, severe oral or pharyngeal mucositis, history of MRSA infection or
colonization.
OR
UÊ-iÛiÀiÊ* Ê>iÀ}ÞÊ­>>« Þ>ÝÃÊÀÊ-ÌiÛià ÃÊ-Þ`Ài®\Ê
Strongly consider allergy consult to verify allergy in patients with
unclear histories (see section on Penicillin allergy, p. 137)
UÊâÌÀi>ÊÓÊ}Ê6Ê+nÊPLUS Gentamicin† (see dosing section, p. 146)
PLUS Vancomycin (see dosing section, p. 150)
†If strong concern for nephrotoxicity and no prior fluoroquinolone use, can substitute
Ciprofloxacin 400 mg IV Q8H for Gentamicin.
Step-down therapy for discharge
UÊÊCiprofloxacin 750 mg PO BID PLUS Amoxicillin/clavulanate 875 mg
PO BID
OR
UÊÝyÝ>VÊ{ääÊ}Ê*"Ê`>Þ
129
6.20 Guidelines for use of antimicrobials in neutropenic hosts
Neutropenic fever
6.20 Guidelines for use of antimicrobials in neutropenic hosts
Persistent fever or new fever after 4-7 days in clinically
stable patients without established bacterial infection
UÊ ÌÕiÊ>ÌLÌVÃÊ>LÛiÊ>`Ê
Ê>ÌvÕ}>ÊVÛiÀ>}iÊ
vÊÀiViÛ}ÊÕV>âiÊ«À« Þ>ÝÃÊÀÊÊvÕ}>Ê«À« Þ>ÝÃ\
UÊV>vÕ}Ê£ääÊ}Ê6Ê+Ó{ÊvÊÃÕÃÊ>`ÉÀÊV iÃÌÊ /ÊÌÊÃÕ}}iÃÌÛiÊ
of fungal infection
OR
UÊ6ÀV>âiÊÈÊ}É}Ê6É*"Ê+£ÓÊÌiÃÊÌÜÊ`ÃiÃÊÌ iÊ{Ê}É}Ê6É
PO Q12H if chest CT suggestive of fungal infection
If receiving Voriconazole or Posaconazole prophylaxis or sinus CT
ÃÕ}}iÃÌÛiÊvÊvÕ}>ÊviVÌ\
UÊ ÃiÁÊxÊ}É}Ê6Ê+Ó{Ê
Clinically unstable patient and/or persistent fever despite
appropriate antibacterial and antifungal coverage
UÊ ÃÕÌÊ"V}ÞÉ/À>ë>ÌÊ Ê
UÊ6>VÞVÊ­ÃiiÊ`Ã}ÊÃiVÌ]Ê«°Ê£xä®ÊPLUS Meropenem 1 g IV
+nÊ´Ê>VÊvÊ«>ÌiÌÊÕÃÌ>LiÊ­ÃiiÊ`Ã}ÊÃiVÌÊ«°Ê£{È®Ê
OR
UÊ-iÛiÀiÊ* Ê>iÀ}Þ\Ê ÃÕÌÊ"V}ÞÉ/À>ë>ÌÊ Ê
130
NOTE:ÊÊ`ÃiÃÊ>ÃÃÕiÊÀ>ÊÀi>ÊvÕVÌÆÊ`ÃiÊ`wV>ÌÃÊ>ÞÊLiÊ`V>Ìi`ÊvÀÊ
reduced CrCI.
1. Leukemia patients
Indication
Agent and dose
Duration
Antibacterial prophylaxis
Moxifloxacin 400 mg PO daily PLUS
Amoxicillin 500 mg PO TID (start on day 5)
Day 1 until
ANC 100/mm3 OR
initiation of
ºÀÃÌÊiÛiÀ»Ê
antibiotics
ÌvÕ}>Ê«À« Þ>ÝÃÊ
Ê
ÀÃÌÊi\Ê6ÀV>âiÊ­ÃiiÊ`Ã}ÊÊ /ÊÃiVÌ®Ê >ÞÊ£ÊÕÌÊÊ
-iV`Êi\Ê*Ã>V>âiÊÃÕëiÃÊÓääÊ}ÊÊ Ê
PO TID OR 300 mg tablet daily
100/mm3
ÌiÀ>ÌÛiÃ\ÊV>vÕ}Ê£ääÊ}Ê6Ê+Ó{Ê",ÊÊ
Ê
Ê
Fluconazole 400 mg PO daily
Antiviral prophylaxis
Ê
* *Ê«À« Þ>ÝÃÊÊ
in high risk patients‡Ê
Ê
Valacyclovir 500 mg PO BID OR Acyclovir
800 mg PO BID
vÊÛÌ}ÊÀÊ`>ÀÀ i>\ÊVÞVÛÀÊÓxäÊ}É2
IV Q12H†
Day 1 until
ANC 100/mm3
ÀÃÌÊi\Ê/*É-8ÊiÊ--ÊÌ>LÊ*"Ê`>ÞÊÊ
-iV`Êi\Ê >«ÃiÊ£ääÊ}Ê*"Ê`>ÞÊ
/ À`Êi\ÊÌÛ>µÕiÊÇxäÊ}Ê*"Ê Ê
>ÞÊ£ÊÕÌÊÊ
ÕÊÊ
ÃÕ«ÀiÃÃÊ
resolves
2. Lymphoma, myeloma patients
Indication
Agent and dose
Duration
Antibacterial prophylaxis
(lymphoma only)
Moxifloxacin 400 mg PO daily
Antifungal prophylaxis
Fluconazole 200 mg PO daily
Day 7 of
chemo until
ANC 500/mm3
Day 1
through all
cycles of
chemotherapy in
high risk
patients.
Antiviral prophylaxis
Valacyclovir 500 mg PO BID OR Acyclovir
800 mg PO BID
vÊÛÌ}ÊÀÊ`>ÀÀ i>\ÊVÞVÛÀÊÓxäÊ}É2
IV Q12H†
Day 7 through
all cycles of
chemotherapy
ÀÃÌÊi\Ê/*É-8ÊiÊ--ÊÌ>LÊ*"Ê`>ÞÊÊ
-iV`Êi\Ê >«ÃiÊ£ääÊ}Ê*"Ê`>ÞÊ
/ À`Êi\ÊÌÛ>µÕiÊÇxäÊ}Ê*"Ê Ê
>ÞÊÇÊÌ ÀÕ} Ê
>ÊVÞViÃÊvÊÊ
V iÊÊ
therapy
Ê
* *Ê«À« Þ>ÝÃÊÊ
in high risk patients‡Ê
Ê
131
6.20 Guidelines for use of antimicrobials in neutropenic hosts
Prophylactic antimicrobials for patients with
expected prolonged neutropenia
6.20 Guidelines for use of antimicrobials in neutropenic hosts
3. Bone marrow transplant patients/peripheral blood stem cell transplant patients
Indication
Agent and dose
Duration
Antibacterial prophylaxis*
Moxifloxacin 400 mg PO daily
Day zero until
engraftment
Antifungal prophylaxis
Fluconazole 400 mg PO daily
Day zero until
ANC 500/mm3
ÌvÕ}>Ê«À« Þ>ÝÃÊÊÊ
patients with GVHD¶
Ê
ÀÃÌÊi\Ê*Ã>V>âiÊÃÕëiÃÊÓääÊ}Ê*"
TID OR 300 mg tablets daily
-iV`Êi\Ê6ÀV>âiÊ­`Ãi`ÊLÞÊÜi} Ì®
69 kg Voriconazole 200 mg PO BID
69 kg to 94 kg Voriconazole 300 mg PO BID
94 kg Voriconazole 400 mg PO BID
Antiviral prophylaxis
Valacyclovir 500 mg PO BID OR
Acyclovir 800 mg PO BID
vÊÛÌ}ÊÀÊ`>ÀÀ i>\ÊVÞVÛÀÊÓxäÊ}É2
IV Q12H †
Day zero
until 1 yr
(allogeneic
transplants)
or 6 months
(autologous
transplants)
Ê
Ê
ÀÃÌÊi\Ê/*É-8ÊiÊ--ÊÌ>LÊ*"Ê`>ÞÊ
-iV`Êi\/*É-8Ê -ÊÌ>LÊÓÊÌiÃÊÜiiÞÊÊ
OR Dapsone 100 mg PO daily
/ À`Êi\ÊÌÛ>µÕiÊÇxäÊ}Ê*"Ê Ê
ÕÀÌ Êi\Ê*iÌ>`iÊÎääÊ}Ê Ê+ÓnÊ`>ÞÃÊ
Ê
Ê
}iiVÊ
ÌÀ>ë>Ì\Ê
Day 21 or
i}À>vÌiÌÊ
­Ü V iÛiÀÊ
is later)
until at least
1 year
(longer if
steroids or
ongoing risk)
Autologous
ÊÌÀ>ë>Ì\Ê
Engraftment
until 6 months
Ê
PCP prophylaxis†Ê
Ê
NOTES:
/*É-8ÊÌ iÀ>«ÞÊÀi`ÕViÃÊÀÃÊvÊviVÌÊÜÌ ÊiV>«ÃÕ>Ìi`ÊL>VÌiÀ>]ÊListeria spp., Nocardia
spp., and Toxoplasmosis, but does not eliminate risk. It is the preferred antibiotic regimen for
PCP prophylaxis.
*In patients with fluoroquinolone allergy or who cannot tolerate a fluoroquinolone due to QTc
prolongation, consider Cefpodoxime 400 mg PO BID.
†Acyclovir should be dosed by ideal body weight
‡Þi>Ê«>ÌiÌÃÊvÊÊÃÌiÀ`ÃÆÊÞ« >Ê«>ÌiÌÃÊvÊ6³]ÊÊV ÀVÊÃÌiÀ`Ã]ÊyÕ`>À>Li°
iÕi>Ê«>ÌiÌÃ\Ê]ÊV ÀVÊÃÌiÀ`Ã]ÊÃÉ«Ê /ÊÕÌÊ£ÊÞi>ÀÊ>vÌiÀÊÌÀ>ë>Ì]ÊÀÊ«>ÌiÌÊÜ Ê
received cladribine, fludarabine, or alemtuzumab.
¬"Ì iÀÊ«À« Þ>ÝÃÊÊ>VÕÌiÊ6 \ÊÝyÝ>V]Ê/*É-8°
132
Filamentous fungi
ID consult recommended for assistance with antifungal selection
TREATMENT
Aspergillus spp.
Initial therapy
UÊÊ6ÀV>âiÊÈÊ}É}Ê6É*"Ê+£ÓÊÌiÃÊÌÜÊ`ÃiÃÊÌ iÊ{Ê}É}Ê6É
PO Q12H (see Voriconazole guidelines, p. 19, for more information).
OR
UÊ Ãi® 5 mg/kg IV Q24H
NOTES:
UÊÊ6ÀV>âiÊÃÊVÃ`iÀi`ÊLÞÊ>ÞÊÌÊLiÊÌ iÊwÀÃÌiÊÌÀi>ÌiÌÊvÊ
suspected filamentous fungal infections in the immunocompromised
host as most of these infections are caused by Aspergillus species.
Although the data are limited, Voriconazole appears more effective
than Amphotericin for this very serious infection.
UÊÊ L>ÌÊ>ÌvÕ}>ÊÌ iÀ>«ÞÊVÃÃÌ}ÊvÊ6ÀV>âiÊPLUS
Micafungin should be considered for the treatment of confirmed
invasive aspergillosis that is documented by culture, positive
galuctomannan assay, or histopathology for the first two weeks
of therapy. Longer duration of combination therapy has not been
evaluated.
Fusarium spp.
UÊÊ ÊVÃÕÌÊà Õ`ÊLiÊÛÛi`ÊÊÌ iÃiÊV>Ãið
UÊÊ6ÀV>âiÊÈÊ}É}Ê6É*"Ê+£ÓÊÌiÃÊÌÜÊ`ÃiÃÊÌ iÊ{Ê}É}Ê
IV/PO Q12H PLUS Ambisome 5 mg/kg IV Q24H (see Voriconazole
guidelines, p. 19, for more information). Dose escalation may be
necessary for some patients.
Scedosporium apiospermum
UÊÊ6ÀV>âiÊÈÊ}É}Ê6É*"Ê+£ÓÊÌiÃÊÌÜÊ`ÃiÃÊÌ iÊ{Ê}É}Ê
IV/PO Q12H PLUS Micafungin 100 mg IV Q24H (see Voriconazole
guidelines, p. 19, for more information).
NOTE:
UÊÊ/Ài>ÌiÌÊÜÌ ÊÌ iÀÊ>}iÌÃÊ >ÃÊÞi`i`Ê`Ã>««Ì}ÊÀiÃÕÌðÊ
Voriconazole appears to be the best option but the data are limited.
133
6.20 Guidelines for use of antimicrobials in neutropenic hosts
Guidelines for the use of antifungal agents in
hematologic malignancy patients
6.20 Guidelines for use of antimicrobials in neutropenic hosts
Zygomycoses (Mucor, Rhizopus, Cunninghamella, etc.).
UÊ Ãi® 5 mg/kg IV once daily PLUS a second antifungal agent
UÊÊ ÊVÃÕÌÊÀiµÕÀi`°
UÊÊ-ÕÀ}V>Ê`iLÀ`iiÌÊ>`ÊVÀÀiVÌÊvÊÕ`iÀÞ}ÊÀÃÊv>VÌÀÃÊ­i°}°Ê
acidosis, hyperglycemia) are critical.
Candida
TREATMENT
UÊÊ9 -/Ê ÊÊ "" Ê 1/1, Ê-"1 Ê 6 ,Ê Ê " - , ÊÊ
CONTAMINANT.
UÊÊ-iiÊÃiVÌÃÊLiÜÊÊi«ÀVÊÌ iÀ>«ÞÊ>`ÊÊ«>Ì }iëiVwVÊ
therapy.
Unspeciated candidemia
UÊV>vÕ}Ê£ääÊ}Ê6Ê+Ó{
OR
UÊ Ãi® 5 mg/kg IV Q24H
If the yeast is C. albicans or C. glabrata, the recommendations for C.
albicans noted below can be followed. If the yeast is not C. albicans,
await speciation before modifying therapy as recommended below.
NOTE: Micafungin does not cover Cryptococcus
Candida albicans
UÊV>vÕ}Ê£ääÊ}Ê6Ê+Ó{
OR
UÊÊ Ãi®ÊÎqxÊ}É}Ê6Ê+Ó{
NOTE: Patients who are clinically stable and no longer neutropenic can
be switched to Fluconazole if the organism is susceptible.
Candida glabrata
UÊV>vÕ}Ê£ääÊ}Ê6Ê+Ó{
OR
UÊÊ Ãi® 5 mg/kg IV Q24H
Candida krusei
UÊV>vÕ}Ê£ääÊ}Ê6Ê+Ó{
OR
UÊÊ Ãi® 5 mg/kg IV Q24H
134
Candida parapsilosis
UÊ Ãi®ÊÎqxÊ}É}Ê6Ê+Ó{Ê
NOTES:
UÊÊÃÌÊC. parapsilosis isolates remain susceptible to Fluconazole, which
can be used in stable and non-neutropenic patients.
UÊÊ/ iÀiÊ>ÀiÊÌi`Ê`>Ì>ÊÌ >ÌÊÃÕ}}iÃÌÊÌ >ÌÊV>vÕ}Ê>ÞÊLiÊviÀÀÊÌÊ
Amphotericin B in these infections.
Candida tropicalis
UÊV>vÕ}Ê£ääÊ}Ê6Ê+Ó{
OR
UÊÊ Ãi®ÊÎqxÊ}É}Ê6Ê+Ó{
TREATMENT NOTES
Hidden Content
- JHH Internal use only
Notes on antifungal susceptibility testing
UÊÊ-ÕÃVi«ÌLÌÞÊÌiÃÌ}ÊvÀÊÕV>âi]ÊÌÀ>V>âi]Ê6ÀV>âi]Ê
Flucytosine (5-FC), and Micafungin is performed routinely on the first
yeast isolate recovered from blood.
135
6.20 Guidelines for use of antimicrobials in neutropenic hosts
A
NOTE: C. krusei is intrinsically resistant to Fluconazole and these
infections can be difficult to treat. In stable patients, Voriconazole can be
used if susceptible and oral therapy is desired. (See p. 19 for dosing).
A
6.20 Guidelines for use of antimicrobials in neutropenic hosts
UÊÊÕV>âiÊ>`ÊV>vÕ}ÊÃÕÃVi«ÌLÌiÃÊ>ÀiÊÀi«ÀÌi`ÊÊ>ÊL`Ê
isolates.
UÊÊ"À}>ÃÃÊÌ >ÌÊ >ÛiÊV>vÕ}Ê ÃÊÊÌ iÊÀ>}iÊvÊ£qÓÊV}ÉÊ
(reported as susceptible) may not respond to treatment. ID consult is
recommended in these cases.
UÊÊSusceptibility testing for conventional Amphotericin B is done routinely
for C. lusitaniae and C. guillemondii and for other organisms by
request.
UÊÊ-ÕÃVi«ÌLÌÞÊÌiÃÌ}ÊÃ Õ`ÊLiÊVÃ`iÀi`ÊÜ i\Ê
UÊÊÕVVÕÌ>iÕÃÊV>``>ÃÃÊÃÊÀivÀ>VÌÀÞÊÌÊÕV>âi
UÊÊ/Ài>Ì}ÊÃÌiÞiÌÃ]Êi}ÌÃ]ÊÀÊi`« Ì >ÌÃÊÜÌ Ê
Fluconazole
UÊÊ `ÊVÕÌÕÀiÃÊ>ÀiÊ«iÀÃÃÌiÌÞÊ«ÃÌÛiÊÊÕV>âi
UÊÊ ÀÕÌiÊÃÕÃVi«ÌLÌÞÊÌiÃÌ}ÊV>ÊLiÊ>ÀÀ>}i`ÊLÞÊV>}ÊÌ iÊ
mycology lab at 5-6148
,iviÀiVi\
-ÊÕ`iiÃÊvÀÊ/Ài>ÌiÌÊvÊ >``>ÃÃ\Ê ÊviVÌÊ ÃÊÓääÆ{n\xäΰ
136
Penicillin reactions – Incidence
UÊÊÊnää¯ÊvÊ«>ÌiÌÃÊÜ ÊÀi«ÀÌÊÌ iÞÊ>Àiʺ>iÀ}V»ÊÌÊ* Ê>VÌÕ>ÞÊ >ÛiÊ
negative skin tests and are not at increased risk of an allergic reaction.
UÊÊ*iVÊÀi>VÌÃÊvÊÃiÊÌÞ«iÊVVÕÀÊÊä°ÇÊÌÊ£ä¯ÊvÊ>Ê«>ÌiÌÃÊ
who get the drug.
UÊÊ 1/\Ê/ iÊV`iViÊvÊ>>« Þ>VÌVÊÀi>VÌÃÊÃÊä°ää{¯ÊÌÊä°ä£x¯°
UÊÊ,>ÌiÃÊvÊVÀÃÃÀi>VÌÊ>iÀ}iÃÊÌÊVi« >ëÀÃÊ>ÀiÊÕÜÊLÕÌÊ
thought to be low.
UÊÊ,>ÌiÃÊvÊ* Ê>`ÊV>ÀL>«iiÊÃÊÌiÃÌÊVÀÃÃÊÀi>VÌÛÌÞÊ>ÀiÊ{ǯ]Ê
although clinical rates of hypersensitivity reactions in patients with
Ài«ÀÌi`Ê* Ê>iÀ}ÞÊÜ ÊÀiViÛiÊV>ÀL>«iiÃÊ>ÀiÊq££¯°
UÊÊ ÀÃÃÊÀi>VÌÃÊÌÊL>VÌ>ÃÊ­âÌÀi>®Ê`Ê "/Ê>««i>ÀÊÌÊVVÕÀ°
Penicillin skin testing
UÊÊ7 iÊ`iÊVÀÀiVÌÞ]ÊÃÊ } ÞÊ«Ài`VÌÛiÊvÊÃiÀÕÃ]Ê>>« Þ>VÌVÊÀi>VÌð
UÊÊ*>ÌiÌÃÊÜÌ Ê>Êi}>ÌÛiÊÃÊÌiÃÌÊ>ÀiÊNOT at risk for anaphylactic reactions.
UÊÊ,>ÀiÞ]ÊÃÊÌiÃÌÊi}>ÌÛiÊ«>ÌiÌÃÊ>ÞÊ}iÌÊ`Ê ÛiÃÊ>`ÊÌV }Ê
following penicillin administration but these RESOLVE with continued
treatment.
UÊÊ-ÊÌiÃÌÃÊV>ÌÊ«Ài`VÌÊ`iÀ>Ì}VÊÀÊÊÀi>VÌÃÊÀÊ`ÀÕ}ÊviÛiÀð
UÊÊ-ÊÌiÃÌ}ÊÃÊÜÊ>Û>>LiÊ>ÌÊ°Ê*i>ÃiÊVÃÕÌÊiÀ}ÞÊ>`Ê
Immunology.
Penicillin reactions—Types
UÊImmediateÊ­ÌÞ«iÊ£®ÊqÊ>« Þ>ÝÃ]Ê Þ«ÌiÃ]Ê>ÀÞ}i>Êi`i>]Ê
wheezing, angioedema, urticaria
UÊÊÃÌÊ>Ü>ÞÃÊVVÕÀÊwithin 1 hour of administration. Hypotension
always occurs soon after administration
UÊÊ >ÊLiÊ«Ài`VÌi`ÊLÞÊÃÊÌiÃÌÃ
UÊAcceleratedÊqÊ>ÀÞ}i>Êi`i>]ÊÜ iiâ}]Ê>}i`i>]ÊÕÀÌV>À>Ê
(NOT hypotension)
UÊÊ"VVÕÀÊÜÌ Ê£ÇÓÊ ÕÀÃÊvÊ>`ÃÌÀ>Ì
UÊÊ >ÊLiÊ«Ài`VÌi`ÊLÞÊÃÊÌiÃÌÃ
UÊLateÊqÊ,>à ʭ>VÕ«>«Õ>ÀÊÀÊÀLvÀÊÀÊVÌ>VÌÊ`iÀ>ÌÌî]Ê
destruction of RBC, WBC, platelets, serum sickness
UÊÊÃÌÊ>Ü>ÞÃÊVVÕÀÊ>vÌiÀÊÇÓÊ ÕÀÃÊvÊ>`ÃÌÀ>Ì
UÊÊ,>à iÃÊÃiÌiÃÊ}Ê>Ü>ÞÊ`iëÌiÊVÌÕi`ÊÌÀi>ÌiÌ
UÊÊ>VÕ«>«Õ>ÀÊ>`ÊÀLvÀÊÀ>Ã iÃÊ "Ê "/Ê«À}ÀiÃÃÊÌÊ
Stevens-Johnson syndrome
UÊÊ>ÌiÊÀi>VÌÃÊ>ÀiÊ "/Ê«Ài`VÌi`ÊLÞÊÃÊÌiÃÌÃ
UÊStevens-Johnson SyndromeÊqÊiÝv>ÌÛiÊ`iÀ>ÌÌÃÊÜÌ ÊÕVÕÃÊ
membrane involvement
137
7.1 Approach to the patient with a history of penicillin allergy
Approach to the patient with a history
of penicillin allergy
7.1 Approach to the patient with a history of penicillin allergy
UÊÊÃÌÊ>Ü>ÞÃÊVVÕÀÊ>vÌiÀÊÇÓÊ ÕÀÃÊvÊ>`ÃÌÀ>Ì
UÊÊ "/Ê«Ài`VÌi`ÊLÞÊ>Ê ÃÌÀÞÊvÊÀ>Ã Ê",ÊLÞÊÃÊÌiÃÌÃ
Approach to the patient with reported penicillin allergy
UÊÊ Àiv]ÊvVÕÃi`Ê ÃÌÀÞÊV>ÊLiÊ6 ,9Ê i«vÕ°
UÊÊ+ÕiÃÌÃÊÌÊ>Ã\
1. How long after beginning penicillin did the reaction occur?
2. Was there any wheezing, throat or mouth swelling, urticaria?
3. If a rash occurred, what was the nature of the rash? Where was it
and what did it look like?
4. Was the patient on other medications at the time of the reaction?
5. Since then, has the patient ever received another penicillin or
Vi« >ëÀÊ­>ÃÊ>LÕÌÊÌÀ>`iÊ>iÃÊi\ÊÕ}iÌ]ÊiyiÝ]Ê
Trimox, Ceftin, Vantin)?
6. If the patient received a beta-lactam, what happened?
Interpreting the history of the patient reporting penicillin allergy
UÊÊANY patient who has a history consistent with an immediate
reaction (laryngeal edema, wheezing, angioedema, urticaria)
SHOULD NOT receive beta-lactams without undergoing skin
testing first EVEN IF they have received beta-lactams with no
problems after the serious reaction.
UÊÊ*>ÌiÌÃÊÜ ÊÀi«ÀÌÊ>>« Þ>VÌVÊÀi>VÌÃÊ>`Ê >ÛiÊÀiViÛi`Ê
other penicillins without problems DO NOT have penicillin allergy
and are not at increased risk for an allergic reaction compared to
the general population.
UÊÊ*>ÌiÌÃÊÜ ÊÀi«ÀÌÊ>>« Þ>VÌVÊÀi>VÌÃÊ>`Ê >ÛiÊÀiViÛi`Ê
cephalosporins can get cephalosporins but not necessarily PCNs.
UÊÊ*>ÌiÌÃÊÜ ÊÀi«ÀÌÊ>Ê ÃÌÀÞÊvÊ>ÊÕÀÌV>À>ÊÀ>Ã ÊÌ >ÌÊÃÊ "/Ê
consistent with Stevens-Johnson syndrome (target lesions with
mucous membrane inflammation) and developed after ≥ 72 hours
of penicillin are not at increased risk for an adverse reaction. They
should, however, be watched closely for development of rashes.
UÊÊ*>ÌiÌÃÊÜ ÊÀi«ÀÌÊÀi>VÌÃÊVÃÃÌiÌÊÜÌ ÊÃiÀÕÊÃViÃÃÊ
(rare) can receive either penicillins or cephalosporins with careful
monitoring for recurrence.
UÊÊ*>ÌiÌÃÊÜ ÊÀi«ÀÌÊÊÃÞ«ÌÃÊ­`>ÀÀ i>]Ê>ÕÃi>®Ê«ÀL>LÞÊ`Ê
not have penicillin allergy and do not appear to be at increased
risk for an adverse reaction. They should be closely observed for
recurrent symptoms and be given supportive therapy if they occur.
,iviÀiViÃ\Ê
ÊÓää£ÆÓnx\Ó{n°
1ÃiÊvÊV>ÀL>«iiÃÊÊ«>ÌiÌÃÊÜÌ Ê*
££xxqÇ°Ê
ÊÌiÀÊi`ÊÓääÇÆ£{È\ÓÈÈq°
138
Ê>iÀ}Þ\ÊÊÌVÀL°Ê
iÌ iÀÊÓää{Æx{\Ê
UÊÊ ÃÕÌÊÌ iÊ ÊÜiLÃÌiÊÀÊÊ«ViÃÊiÊ­*"®Ê­ÜÜÜ°
hopkinsmedicine.org/heic) for detailed isolation charts, HEIC policies,
and surveillance information
Hand hygiene
UÊÊvÊ >`ÃÊ>ÀiÊÌÊÛÃLÞÊÃi`]ÊÌ iÊ>V L>Ãi`Ê >`ÊÃ>ÌâiÀÃÊ>ÀiÊ
recommended for cleaning. If hands are visibly soiled, wash hands
with soap and water for at least 15 seconds.
UÊÊ>`Ê Þ}iiÊÃÊÀiµÕÀi`ÊÕ«ÊiÌiÀ}Ê>Ê«>ÌiÌÊÀ]ÊÕ«ÊiÝÌ}]Ê
between patients in a semi-private room, and other times per hospital
policy.
UÊÊ1ÃiÊÃ>«Ê>`ÊÜ>ÌiÀÊÕ«Êexiting the room of a patient with
C. difficile infection.
UÊÊ Ê>ÀÌwV>Êw}iÀ>ÃÊ>ÀiÊ«iÀÌÌi`ÊvÀÊ>ÞÊÃÌ>vvÊiLiÀÊÜ Ê >ÃÊ
patient contact or handles sterile supplies.
Bloodborne pathogen exposures (needlestick or other exposure)
The prompt treatment of injuries and exposures is vital to prevent the
transmission of disease. Whatever the exposure, IMMEDIATE cleaning of
the exposure site is the first priority.
UÊÊ-ÊÜÕ`ÃÊÃ Õ`ÊLiÊVi>i`ÊÜÌ ÊÃ>«Ê>`ÊÜ>ÌiÀ
UÊÊÕVÕÃÊiLÀ>iÃÊÃ Õ`ÊLiÊyÕÃ i`ÊÌ ÀÕ} ÞÊÜÌ ÊÜ>ÌiÀ
UÊÊ ÞiÃÊÃ Õ`ÊLiÊÀÀ}>Ìi`ÊÜÌ Ê>ÊÌiÀÊvÊÀ>ÊÃ>i
vÌiÀÊVi>}ÊÌ iÊiÝ«ÃÕÀiÊÃÌi]ÊV>Êx-/8Ê­xÇn{®Ê>`ÊvÜÊ
instructions to contact the ID physician. Workplace injuries should be
Ài«ÀÌi`Êi`>ÌiÞÊÊÌ iʺ «ÞiiÊ,i«ÀÌÊvÊV`iÌÊÀ»Ê>`Ê
to the Occupational Injury ClinicÊ­ >VÊ£Î]Ê`>ÞqÀ`>Þ]ÊÇ\ÎäÊ
a.m. to 4 p.m., 5-6433), and to your supervisor.
Standard Precautions
UÊÊ,ÕÌiÊ >`Ê Þ}iiÊ
UÊÊ ÃÃÌiÌÊ>`ÊVÀÀiVÌÊ}ÛiÊÕÃiÊÊ
UÊÊ >}ÊVÌ>>Ìi`ÊiÊ>ÌÊ«ÌÊvÊÕÃi
UÊÊ,i}Õ>ÀÊVi>}ÊvÊiÛÀiÌ>Ê
surfaces
UÊ««À«À>ÌiÊÕÃiÊvÊ}ÜÃÊÌÊ«ÀiÛiÌÊÊ UÊ,ÕÌiÊVi>}ÊÀÊ`ëÃ>Êv
contamination of uniform/clothing
patient-care equipment
UÊ««À«À>ÌiÊÕÃiÊvÊ>ÃÃ]ÊiÞiÊÊ
UÊ-ÌÀVÌÊ>` iÀiViÊÌ
protection and face shields (i.e., when
occupational safety requirements
suctioning, or when splash likely)
139
8.1 Hospital Epidemiology & Infection Control
A
Hospital Epidemiology and Infection Control
(HEIC)
8.1 Hospital Epidemiology & Infection Control
A
Communicable diseases—exposures and reporting
ÊÃ Õ`ÊLiÊÌwi`\
UÊÊvÊ«>ÌiÌÃÊÀÊ 7ÃÊ>ÀiÊiÝ«Ãi`ÊÌÊ>ÊVÕV>LiÊ`Ãi>ÃiÊ­°i°Ê
meningococcal disease, varicella, TB etc.)
UÊÊLÕÌÊ 7ÃÊÜÌ Ê>VÕÌiÊ i«>ÌÌÃÊ]Ê ÊÀÊ ]Ê->i>]Ê- }i>]Ê
Campylobacter, or pneumonia requiring hospital admission
UÊÊLÕÌÊ>ÞÊÕÕÃÕ>ÊVVÕÀÀiViÊvÊ`Ãi>ÃiÊÀÊVÕÃÌiÀ]Ê«>ÀÌVÕ>ÀÞÊ
diseases that have the potential to expose many susceptible
individuals
UÊÊ-ÕëVÊÀÊ`>}ÃiÃÊvÊÌ iÊvÜ}Ê`Ãi>ÃiÃÊ­`Ãi>ÃiÃÊÜÌ Ê
require immediate notification by phone or pager). If disease is
in a HCW, notify HEIC and Occupational Health (98 N. Broadway,
-ÕÌiÊ{Ó£]Ê`>ÞqÀ`>Þ]ÊÇ\ÎäÊ>°°ÊÌÊ{\ääÊ«°°]ÊxÈÓ££®Ê
immediately
Anthrax Avian Influenza Botulism Brucellosis
Creutzfeldt-Jakob disease (CJD) Diphtheria Glanders Highly resistant organisms (i.e. VISA,
VRSA) Legionellosis
Measles (rubeola) Meningococcal disease Monkeypox Mumps
Pertussis Plague Poliomyelitis
Q Fever
Rabies Ricin toxin Rubella (German measles)
Salmonellosis
SARS Scabies
Shigellosis
Smallpox (orthopox viruses) Streptococcal Group A or B invasive
disease Tuberculosis Tularemia Varicella (chickenpox or disseminated
zoster) Viral hemorrhagic fever Yellow Fever Physicians are required to report communicable disease to the
>ÌÀiÊ ÌÞÊi>Ì Ê i«>ÀÌiÌÊ­{£äÎÈ{{ÎÈ]Êv>Ý\Ê{£äÈÓxäÈnn®°Ê
For a complete list of communicable diseases, see the HEIC Web site,
Ì iÊ Ê7iLÊÃÌi]Ê ÌÌ«\ÉÉ`i >°` °>ÀÞ>`°}ÛÉ-Ìi*>}iÃÉÜ >Ì
to-report.aspx or the BCHD Web site, www.baltimorehealth.org/acd.
html.
140
141
To enter room
MRSA, C.diff, zoster§
Door closed
Mask/Eye Protection
Gown and Gloves
Examples
Droplet
Precautions
(orange)
Required unless
cohorted*
No
If within 6 feet
of patient
To enter room
Influenza, bacterial
meningitis
Yes
PAPR or N95† to
enter room‡
No
TB, disseminated
zoster§
Airborne
Precautions
(blue) ¶
Required
8.2 Infection control precautions
* Required for pertussis and diphtheria
† Fit-testing is required to use an N95 mask for airborne precautions
‡ HCWs who are Varicella-immune do not have to wear a PAPR or N95 if patient is in isolation for zoster or chickenpox
§ Disseminated zoster, zoster in an immunocompromised host, and chickenpox require both Contact and Airborne Precautions
(sign color)
Private room
Contact
Precautions
(pink)
Required unless
cohorted
No
No
JHH Precautions Categories
These precaution categories must be used in addition to Standard Precautions. The following table includes general requirements for precaution
categories. The complete table and the type of isolation required for each organism can be found on the HEIC website. If recommendations on this table
cannot be followed, please contact HEIC.
8.3 Disease-specific infection control recommendations
Disease-specific infection control
recommendations
Carbapenem-resistant Enterobacteriaceae (CRE)
Routine active surveillance cultures for CRE are performed in patients
who have been hospitalized in a country other than the U.S. in the past
6 months. Patients are placed on Contact Precautions pending cullture
results. The results are to be used for isolation purposes, not to guide
therapy or clinical care. The overwhelming majority of positive
surveillance cultures represents colonization, not infection, and
should not prompt any antimicrobial therapy.
Creutzfeldt-Jakob disease (CJD)
CJD, variant CJD and other diseases caused by prions are resistant to a
number of standard sterilization and disinfection procedures. Iatrogenic
transmission of CJD has been associated with percutaneous exposure
to medical instruments contaminated with prion/central nervous system
(CNS) tissue residues, transplantation of CNS and corneal tissues and
recipients of human growth hormone and gonadotropin. Transmission of
CJD has not been associated with environmental contamination or from
person-to-person via skin contact. The following additional precautions
must be made when processing equipment that could be contaminated
ÜÌ Ê«ÀÊÀi>Ìi`Ê>ÌiÀ>\
UÊÊ ÌvÞÊ Ê>`ÊÌ iÊÕÌÊ>>}iÀÉV >À}iÊÕÀÃiÊi`>ÌiÞÊvÊ>ÞÊ
suspected or confirmed CJD case and refer to the CJD policy on the
HEIC Web site.
UÊÊ1ÃiÊ`ëÃ>LiÊiµÕ«iÌÊÜ iiÛiÀÊ«ÃÃLi°ÊvÊ`ëÃ>LiÊ
equipment is used, Central Sterile Department shall be notified prior to
the start of the procedure.
UÊÊ>LiÊ>Ê>LÀ>ÌÀÞÊ>`Ê«>Ì }ÞÊÀiµÕÃÌÃÊ>ÃÊÃÕëiVÌi`Ê Ê>`Ê
notify the lab before sending specimens.
UÊÊ/ iÊvÜ}Ê>ÀiÊVÃ`iÀi`Ê } ÞÊviVÌÛiÊ>`ÊÃ Õ`ÊLiÊ >`i`Ê
ÜÌ ÊiÝÌÀiiÊV>ÕÌ\ÊLÀ>]Êë>ÊVÀ`]Ê«ÌVÊÌÃÃÕiÃÊ>`Ê«ÌÕÌ>ÀÞÊ
gland
UÊÊ/ iÊvÜ}Ê>ÀiÊVÃ`iÀi`ÊÌÊLiÊvÊÜiÀÊviVÌÛÌÞ\Ê -]Ê`iÞ]Ê
liver, lung, lymph nodes, spleen, placenta, tonsillar tissue and olfactory
tissue.
Methicillin-resistant Staphylococcus aureus (MRSA)
Routine active surveillance cultures for MRSA are performed on select
units to identify patients with MRSA. When a culture is positive for
MRSA the patient is placed on Contact Precautions. The results are
to be used for isolation purposes, not to guide therapy or clinical care.
The overwhelming majority of positive surveillance cultures
142
Surveillance cultures should be obtained upon admission and weekly
ÊÌ iÊvÜ}ÊÕÌÃ\Ê 1]Ê7 1]Ê 6- 1]Ê- 1]Ê /1Ê­7®]Ê
1]Ê
CCU/PCCU, PICU, NICU, oncology units, Nelson 4.
To remove a patient from MRSA precautions, cultures from the original
site of infection and 2 nares cultures taken ≥ 72 hours apart must be
negative. Nares cultures should not be sent if the patient has received
antibiotics active against MRSA in the previous 48 hours. Once this is
accomplished, call HEIC to review culture data and initiate deflagging.
Pertussis
All patients with pertussis should be placed on Droplet Precautions
for five days from the start of therapy. If the patient is not on therapy,
Droplet Precautions should be continued for three weeks from the onset
of cough. Private room is required.
/Ài>ÌiÌ\
UÊÊâÌ ÀÞVÊxääÊ}Ê*"ÊViÊÊ`>ÞÊ£]ÊÌ iÊÓxäÊ}Ê*"Ê`>ÞÊÊ
`>ÞÃÊÓqx
OR
UÊÊ>VÀ`iÊ>iÀ}Þ\Ê/*É-8Ê£Ê -ÊÌ>LiÌÊ*"Ê ÊvÀÊ£{Ê`>ÞÃ
Prophylaxis with the above regimens is required for all household
contacts within three weeks of exposure. Use the same antibiotic as
for treatment. All household contacts and HCWs with exposure to
the patient should also have up-to-date immunizations for Bordetella
pertussis.
Scabies
All patients with conventional or Norwegian scabies should be placed on
Contact Precautions. Norwegian scabies is a severe form of heavy
mite infestation.
UÊÊ*ÀÛ>ÌiÊÀÊÀiµÕÀi`°
UÊÊ*>ÌiÌÃÊÜÌ ÊVÛiÌ>ÊÃV>LiÃÊÕÃÌÊLiÊÌÀi>Ìi`ÊÜÌ Ê>ÊÃV>LV`iÊ
once, and the precautions may be discontinued 24 hours after the
treatment is completed.
UÊÊ*>ÌiÌÃÊÜÌ Ê ÀÜi}>ÊÃV>LiÃÊÀiµÕÀiÊÓÊÌÀi>ÌiÌÃÊÜÌ Ê>ÊÃV>LV`iÊ
1 week apart. Contact precautions may be discontinued 24 hours
after the second treatment is completed.
UÊÊviÃÌi`ÊVÌ }Ê>`ÊiÊÃ Õ`ÊLiÊÃi>i`ÊÊ>Ê«>ÃÌVÊL>}ÊvÀÊ{nÊ
hours. The mite will not survive off a human host for more than 48
hours. Clothing/patient belongings should be sent home with the
patient’s family/caretaker. Linens and clothing should be washed in
the washing machine on the hot cycle.
143
8.3 Disease-specific infection control recommendations
represents colonization, not infection, and should not prompt
any antimicrobial therapy.
8.3 Disease-specific infection control recommendations
UÊÊvÊ«À}i`ÊÃÌÃÊVÌ>VÌÊVVÕÀÃÊÜÌ Ê>ÊÃV>LiÃÊ«>ÌiÌ]Ê
prophylactic treatment is required. Healthcare workers should contact
HEIC if an exposure is suspected.
Vancomycin-resistant enterocci (VRE)
Routine active surveillance cultures for VRE are performed on select
units to identify patients with VRE. Surveillance culture results are found
ÊÌ iÊiiVÌÀVÊ«>ÌiÌÊÀiVÀ`ÊÜÌ ÊÌ iÊÌiÃÌÊ>iʺ >VÌiÀ}Þ-Ì
6, Ê-ÌÊ-ÕÀÛ°Ê ÕÌ°»Ê7 iÊ>ÊVÕÌÕÀiÊ}ÀÜÃÊ6, ]ÊÌ iÊ«>ÌiÌÊÃÊy>}}i`Ê
for Contact Precautions. The results are to be used for isolation
purposes, not to guide therapy or clinical care. The overwhelming
majority of positive surveillance cultures represents colonization,
not infection, and should not prompt any antimicrobial therapy.
Surveillance cultures should be obtained upon admission and weekly
ÊÌ iÊvÜ}ÊÕÌÃ\Ê 1]Ê7 1]Ê 6- 1]Ê- 1]Ê /1Ê­7®]Ê /Ê>`Ê
Leukemia units, NCCU, PICU.
The patient must be off antibiotics for ≥ 48 hours and cultures from
original site of infection AND 3 stool or perirectal cultures taken ≥ 1
week apart must be negative. Once this is accomplished, call HEIC to
review culture data and initiate deflagging.
Varicella-Zoster
Immunocompetent patients with disseminated zoster and all
immunosuppressed patients with zoster need Contact AND Airborne
Precautions°Ê/ iÊvÜ}Ê`iwÌÃÊ>««ÞÊÌÊ«>ÌiÌÃÊÜÌ ÊâÃÌiÀ\
UÊÊImmunosuppressed:ÊLiÊ>ÀÀÜÊÌÀ>ë>ÌÊÜÌ ÊÌ iÊ«>ÃÌÊÞi>ÀÆÊ
>VÕÌiÊiÕi>ÆÊÃ`ÊÀ}>ÊÌÀ>ë>ÌÊÀiV«iÌÃÆÊ«>ÌiÌÃÊÀiViÛ}Ê
cytotoxic or immunosuppressive treatments, including steroid
treatment for ≥ ÎäÊ`>ÞÃÊÜÌ ÊÌ iÊvÜ}Ê`ÃiÃ\Ê`iÝ>iÌ >ÃiÊ
3 mg daily, cortisone 100 mg daily, hydrocortisone 80 mg daily,
«Ài`ÃiÊÓäÊ}Ê`>Þ]ÊiÌ Þ«Ài`ÃiÊ£ÈÊ}Ê`>ÞÆÊ6³Ê«>ÌiÌÃÊ
with CD4 < 200
UÊÊDisseminated: lesions outside of 2 contiguous dermatomes
144
Aminoglycoside dosing weight:
Calculate Ideal Body Weight (IBW)
IBW female (kg)ÊrÊ(2.3 x inches over 5’)ʳÊ45.5
IBW male (kg) r (2.3 x inches over 5’)ʳÊ50
For patients < 20% over IBW, use Actual Body Weight
(ABW)
For patients ≥ 20% over IBW, use Dosing Body Weight
(DBW)
­ 7®ÊrÊQ 7ʳÊä°{Ê­ 7ÊqÊ 7®RÊ
Estimation of creatinine clearance (CrCl) by Cockcroft-Gault
equation:
(If a patient’s renal function is declining, this equation may overestimate CrCl)
Ê
À Êr ­£{äÊqÊ>}i®Ê­Üi} ÌÊÊ}I® x 0.85 (if female)
72 (serum creatinine)
* Use Actual Body Weight (ABW) unless patient ≥ÊÓä¯ÊÛiÀÊ 7]ÊÕÃiÊ
above
7Ê>ÃÊ`iÃVÀLi`Ê
Extended-interval dosing, also sometimes referred to as “oncedaily” administration, utilizes higher dose and less frequent
aminoglycoside administration, whereas patient-specific dosing,
previous referred to as “traditional dosing”, typically utilizes smaller
doses with more frequent administration. See table below for dosing
recommendation based on indication and patient’s renal function. For
mycobacterial infections, urinary tract infections, SICU/WICU
protocol and gram-positive synergy (e.g. endocarditis), please
see separate sections below. For cystic fibrosis patients, see the
Cystic Fibrosis section (p.92)
145
A. Aminoglycoside dosing and monitoring
A
Aminoglycoside dosing and monitoring
Aminoglycosides enhance the efficacy of some antibiotics. Except for
urinary tract infections, aminoglycosides should seldom be used alone
to treat infections.
A. Aminoglycoside dosing and monitoring
A
Aminoglycoside dosing for Gram-negative
infections
IndicationsÊ
DosingÊ
Ê
Ê
Ê
Ê
Ê
Ê
Ê
Ê
Patient-specific dosing
,i>Êv>ÕÀi]ÊÊ É 66 ]Êi`V>À`ÌÃ]ÊÊ
Gram-negative infections (in combination with
beta-lactams), CNS infections, septic shock,
burn patients, patients with altered volume
status (e.g. ascites, anasarca, trauma)
Ê ÃiÊ­}®ÊrÊ`iÃÀi`Ê«i>ÊÝÊQ7i} ÌÊ­}®ÊÝÊ6`ÊÊ
­É}®RÊ
UÊÊ iÃÀi`Ê«i>\ choose from below
UÊÊ7i} Ì\ ABW or DBW
UÊÊVolume of distribution (Vd) typically ranges
LiÌÜiiÊä°ÓxÊqÊä°xÊÉ}ÊÊÃÌÊ«>ÌiÌðÊ
Higher Vd should be used in critically ill and
volume overloaded
patients.
Extended-interval dosing
UÊÊ À>ÊÀi>ÊvÕVÌÊ­ À Ê
>60 mL/min) and all other
indications not listed under
patient specific dosing
iÌ>VÉ/LÀ>ÞV\
xÇÊ}É}Ê6Ê+Ó{
>V\
15-20 mg/kg IV Q24H
Ã}ÊÌiÀÛ>ÊL>Ãi`ÊÊ À \
À ÊÈä\Ê+nI
À ÊÎäÈä\Ê+£Ó
À ÊÎäÉ 66 É \Ê`ÃiÊLÞÊiÛi
*If targeting high peaks, use maintenance dose
frequency of Q12-24H.
Desired
Peaks and
Troughs
Peak
Pneumonia
Septic shock
Endocarditis
Osteomyelitis
MDR
organismsÊ
Trough
Gentamicin/
Tobramycin
10 mcg/mL
Amikacin
8-10 mcg/mL
20-30 mcg/mL
25-35 mcg/mL
This dosing strategy is designed
ÌÊÌ>À}iÌÊÌ iÊvÜ}\
Peak
iÌ>VÉ/LÀ>ÞV\Ê£ÈÓä
mcg/mL
>V\Ê{äÈäÊV}É
Trough
iÌ>VÉ/LÀ>ÞV\Ê
£ÊV}É
>V\Ê{ÊV}É
10-20 mcg/mL 45-50 mcg/mL
L>Ãi`ÊÊ Ê L>Ãi`ÊÊ Ê
Gentamicin/ Amikacin
Tobramycin
All IndicationsÊ £ÓÊV}ÉÊ £äÊV}É
Therapeutic Trough: draw 30 minutes prior to the 3rd dose If the patient meets ANY of the
Drug
criteria below, a trough level
Monitoring Peak: obtain 1 hour after end of infusion, after is recommended prior to the
the 3rd dose.
Ó`Ê`Ãi\
UÊÊ VÌ>ÌÊi« ÀÌÝVÊ
Frequency of monitoring
medications
Ê
UÊÊ"ViÊ>ÊÜiiÊ>vÌiÀÊ`iÃÀi`Ê«i>ÉÌÀÕ} ÊÃÊ
UÊÊ ÌÀ>ÃÌÊiÝ«ÃÕÀiÊ
established in patients with normal renal
UÊ}iÊ≥ 60 years
function
UÊÊ*>ÌiÌÊÃÊÊÌ iÊ 1
Ê
UÊÊÀiÊÌ >ÊViÊÜiiÞ\Ê
UÊÊ"Ì iÀÊÀÃÃÊvÀÊi« ÀÌÝVÌÞÊ
After changes in dosing regimen
­i°}°Ê`>LiÌiÃ]Ê`iÞÊ/8®
Patient is on dialysis
If trough higher than desired
Patient in acute renal failure, SCr increased troughs, use patient specific
LÞÊä°xÊ}É`ÊÀÊÎä¯vÀÊL>ÃiiÊ
dosing to adjust dose.
Major changes in the patient’s volume status
146
Amikacin is the preferred agent to treat all mycobacterial infections,
except Mycobacterium chelonae. For M. chelonae infections,
Tobramycin is the recommended aminoglycoside. Streptomycin
is another aminoglycoside sometimes used to treat mycobacterial
infections such as M. tuberculosis. Please contact the Antimicrobial
Stewardship Program pharmacist for Tobramycin/Streptomycin dosing
recommendation for this indication.
Amikacin:
À>ÊÀi>ÊvÕVÌ\
"ViÊ`>Þ\Ê£xÊ}É}Ê6Ê+Ó{Ê­ÀÊ£äÊ}É}Ê6Ê+Ó{ÊvÊxäÊÞi>ÀÃÊvÊ
age)
/ ÀViÊÜiiÞ\ÊÓxÊ}É}Ê6ÊÌ ÀiiÊÌiÃÊ>ÊÜiiÊ­>ÞÊLiÊÀiÊ`vwVÕÌÊ
to tolerate)
LÀ>ÊÀi>ÊvÕVÌ\ Discuss with pharmacy clinical specialist
Therapeutic drug monitoring: Peak and trough not generally
iViÃÃ>ÀÞ]ÊiÝVi«ÌÊÊÌ ÃiÊÜÌ ÊÀi>ÊÃÕvwViVÞÊ­,ÊÈäÊÉ®Ê
>`ÊvÊ- ÀÊVÀi>ÃiÃÊLÞÊä°xÊ}É`ÊÀÊÎä¯ÊvÀÊL>ÃiiÊÜ iÊ«>ÌiÌÊ
on aminoglycoside therapy. Check a trough concentration to monitor for
toxicity. Peaks in the low 20 mcg/mL range are acceptable, and trough
VViÌÀ>ÌÃÊ>ÀiÊ«ÀiviÀ>LÞÊ{ÊVÉÊÀÊÕ`iÌiVÌ>Li°
Aminoglycoside dosing in urinary tract infections
CrCl (mL/min)
≥60
40-59
20-39
ÓäÊ
Gentamicin/Tobramycin
3 mg/kg IV Q24H or
1 mg/kg IV Q8H
1 mg/kg Q12H
1 mg/kg Q24H
£Ê}É}Ê"
IÊ
Amikacin
10 mg/kg IV Q24H or
3 mg/kg IV Q8H
3 mg/kg IV Q12H
3 mg/kg IV Q24H
ÎÊ}É}Ê6Ê"
I
*Give one dose, check level in 24 hours, redose when Gentamicin/Tobramycin level
£ÊV}ÉÊÀÊ>VÊ{ÊV}É
}ÞVÃ`iÃÊ>ÀiÊ } ÞÊVViÌÀ>Ìi`ÊÊÕÀiÆÊÌ iÀivÀi]ÊÌ iÀ>«iÕÌVÊ
drug monitoring is not necessary in patients with normal renal function.
Suggested doses in the above table will likely provide adequate urine
concentrations for highly susceptible organisms. Trough should be
checked to monitor for toxicity in patients with renal insufficiency
­,ÊÈäÊÉ®Ê>`ÊvÊ- ÀÊVÀi>ÃiÃÊLÞÊä°xÊ}É`ÊÀÊÎä¯ÊvÀÊ
baseline while patient on aminoglycoside therapy.
UÊÊGentamicin/Tobramycin:Ê`iÃÀi`ÊÌÀÕ} Ê£ÊV}ÉÊÀÊÕ`iÌiVÌ>Li°Ê
UÊÊAmikacin:Ê`iÃÀi`ÊÌÀÕ} Ê{ÊV}ÉÊÀÊÕ`iÌiVÌ>Li°
147
A. Aminoglycoside dosing and monitoring
A
Aminoglycoside dosing in mycobacterial
infections
A. Aminoglycoside dosing and monitoring
A
Aminoglycoside dosing in the SICU/WICU
Gentamicin/Tobramycin
Loading dose 4 mg/kg using actual body weight, followed by a
patient-specific maintenance dose.
Amikacin
Loading dose 16 mg/kg using actual body weight, followed by a
patient-specific maintenance dose.
Therapeutic Drug Monitoring
vÌiÀÊ>`}Ê`Ãi\Ê£Ê ÕÀÊ«i>Ê>`ÊnÊ ÕÀÊiÛiÊ>vÌiÀÊÌ iÊi`ÊvÊÌ iÊ
infusion to facilitate calculating patient specific kinetic parameters.
Aminoglycoside dosing for Gram-positive synergy
Dosing for patients with normal renal function:
UÊGentamicin\ÊÎÊ}É}Ê6ÊViÊ`>ÞÊÃÊÀiVi`i`ÊvÀÊÌÀi>ÌiÌÊ
of endocarditis with Viridans streptococci or S. bovis in patients with
normal renal function (CrCl 60 ml/min).
UÊÊGentamicin: 1 mg/kg IV Q8H is recommended for treatment
Enterococcal and other Gram-positive endocarditis infections in
patients with normal renal function (CrCl 60 ml/min). Patients >65
years old should be started on Q12H if normal renal function.
Dosing adjustment for renal insufficiency
CrCl (mL/min)
{äqxÊÊ
ÓäqÎÊÊ
ÓäÊ
Dosing
£Ê}É}Ê+£Ó
£Ê}É}Ê+Ó{
£Ê}É}Ê"
I
IÊÊÛiÊiÊ`Ãi]ÊV iVÊiÛiÊÊÓ{Ê ÕÀÃ]ÊÀi`ÃiÊÜ iÊiÛiÊ£Ê}É
NOTE: See infective endocarditis guidelines (p. 65) for duration.
THERAPEUTIC DRUG MONITORING
UÊÊ*i>Ê>`ÊÌÀÕ} Ê>ÀiÊÀiVi`i`Ê>ÀÕ`ÊÌ iÊÌ À`Ê`ÃiÊÌÊ>ÃÃÕÀiÊ
appropriate dosing.
UÊÊ iÃÀi`ÊÃiÀÕÊVViÌÀ>ÌÃÊvÊGentamicin
Peak levels:ÊÎqxÊV}É
Trough levels:ÊÊ£ÊV}É
148
NEPHROTOXICITY
UÊÊSerum creatinine should be measured at least every other day. If
VÀi>ÌiÊVÀi>ÃiÃÊLÞÊä°xÊ}É`ÊÀÊÎä¯ÊvÀÊL>Ãii]ÊÕÃiÊ«>ÌiÌÊ
specific dosing.
UÊÊi>ÃÕÀiÊserum aminoglycoside levels as needed. See each dosing
section above for frequency.
UÊÊ-iÊ`>Ì>ÊÃÕ}}iÃÌÊÌ >ÌÊÜiÃÌÊiÛiÊvÊi« ÀÌÝVÌÞÊVVÕÀÃÊÜ iÊ
aminoglycosides are administered during the activity period (e.g.
£Î\Îä®]ÊÌ iÀivÀiÊ>vÌiÀÊ>`ÃÌÀ>ÌÊÃÊ«ÀiviÀÀi`°Ê
OTOTOXICITY
UÊÊ Ã`iÀÊLÜiiÞÊVV>ÊÃVÀii}ÊvÀÊÌÌÝVÌÞ
Ê UÊÊ iVÊL>ÃiiÊÛÃÕ>Ê>VÕÌÞÊÕÃ}Ê>Ê-iiÊ«ViÌÊV>À`
Ê UÊÊ/ÊÃVÀiiÊvÀÊÌÌÝVÌÞ]Ê >ÛiÊ«>ÌiÌÊà >iÊ i>`Ê>`ÊÌ iÊÀiÀi>`Ê
card.
Ê UÊÊ ViÀÊà Õ`ÊLiÊÀ>Ãi`ÊvÊ«>ÌiÌÊÃiÃÊÓÊiÃÊvÊÛÃÕ>Ê>VÕÌÞ°Ê
Consider formal audiology testing.
Ê UÊÊ Ì>VÌÊÕ`}ÞÊ­xÈ£xήÊvÀÊ i«ÊÜÌ ÊÌiÃÌ}ÊvÀÊÌÌÝVÌÞ
,iviÀiViÃ\
*É* Ê«>À>iÌiÀ\ÊÊviVÌÊ ÃÊ£nÇÆÊ£xx\Îq
"ViÊ`>ÞÊ}À>ÃÊÀiÛiÜ\ÊPharmacotherapy ÓääÓÆÊÓÓ­®\£äÇÇq£änΰ
*>ÌiÌëiVwVÊ`Ã}\ÊCrit Care MedÊ££ÆÊ£\£{näq£{nx°
- 1É7 1Ê`Ã}\ÊSurgeryÊ£nÆÊ£Ó{\ÇÎn°
i« ÀÌÝVÌÞ\ÊAntimicrob Agents and ChemotherÊÓääÎÆÊ{Ç\£ä£ä°
/-É -ÊÞVL>VÌiÀÕÊÕ`iiÃ\ÊAm J Respir Crit Care MedÊÓääÇÆÊ£Çx\ÎÈÇq{£È°
À>«ÃÌÛiÊ-ÞiÀ}Þ\ÊCirculationÊÓääxÆÊ£££­Óή\ÊiÎ{Êq{Î{°
149
A. Aminoglycoside dosing and monitoring
A
Monitoring for toxicity for inpatients
B. Vancomycin dosing and monitoring
A
Vancomycin dosing and monitoring
DOSING
£°Ê ÃÌ>ÌiÊVÀi>ÌiÊVi>À>ViÊ­ À ®ÊÕÃ}Ê VVÀvÌ>ÕÌÊiµÕ>Ì\
À Êr
­£{äÊqÊ>}i®Ê­Üi} ÌÊÊ}®Ê
72 (serum creatinine*)
x 0.85 (if female)
* For patients with low muscle mass (i.e. many patients > 65 yrs), some advocate using
a minimum value of 1 to avoid overestimation of CrCl
2. Patients who are seriously ill with complicated infections such as
meningitis, pneumonia, osteomyelitis, endocarditis, and
bacteremia and normal renal function should receive initial loading
dose of 20-25 mg/kg, followed by 15-20 mg/kg Q8-12H using
Actual Body Weight (ABW). For other indications see nomogram
dosing below.
3. Calculate maintenance dose (using ABW) based on estimated or
actual CrCl. See suggested nomogram dosing below.
Note: Younger patients with normal renal function may need higher or
more frequent dosing than suggested below.
Weight
(kg)
{äÊ
{äqÈäÊ
>60
30–59
Consult Pharmacy
ÇxäÊ}Ê
ÇxäÊ}ÊÊ
Q12H
Q24H
ÈäqÇxÊ £äääÊ}Ê £äääÊ}ÊÊ
Q12H
Q24H
ÇxqäÊ £ÓxäÊ}Ê £ÓxäÊ}ÊÊ
Q12H
Q24H
äq££äÊ £xääÊ}Ê £xääÊ}ÊÊ
Q12H
Q24H
££äq£ÓxÊ £ÇxäÊ}Ê £ÇxäÊ}ÊÊ
Q12H
Q24H
£Óxq£{äÊ ÓäääÊ}Ê ÓäääÊ}ÊÊ
Q12H
Q24H
>140
Consult Pharmacy
CrCl (mL/min)
15–29
<15
ÇxäÊ}Ê
Q48H
£äääÊ}Ê
Q48H
£ÓxäÊ}Ê
Q48H
£xääÊ}Ê
Q48H
£ÇxäÊ}Ê
Q48H
ÓäääÊ}Ê
Q48H
£äääÊ}]ÊÌ iÊÀi`ÃiÊLÞÊiÛi†
£äääÊ}]ÊÌ iÊÀi`ÃiÊLÞÊiÛi†
£ÓxäÊ}]ÊÌ iÊÀi`ÃiÊLÞÊiÛi†
£xääÊ}]ÊÌ iÊÀi`ÃiÊLÞÊiÛi†
£ÇxäÊ}]ÊÌ iÊÀi`ÃiÊLÞÊiÛi†
ÓäääÊ}]ÊÌ iÊÀi`ÃiÊLÞÊiÛi†
ÀÊ«>ÌiÌÃÊÜÌ Ê À Ê£xÊÉÊ>`ÊÌÊÀiViÛ}Ê i`>ÞÃÃÊÀi`ÃiÊÜ iÊÀ>`Ê
iÛiÊ£xqÓäÊV}É°Ê
†
DOSING IN RENAL REPLACEMENT THERAPY
Dosing is dependent on type of renal replacement therapy.
Intermittent Hemodialysis (iHD)
UÊInitial dose: 15-20 mg/kg once
UÊÊ*>ÌiÌÃÊÃ Õ`ÊLiÊÀi`Ãi`ÊL>Ãi`ÊÊÃiÀÕÊiÛiÃÊ`À>ÜÊ>ÀÕ`ÊÌ iÊ
dialysis session. Consider redosing at 5-10 mg/kg.
150
Continuous Renal Replacement Therapy (e.g. CVVHD)
UÊLoading dose: 25-30 mg/kg once
UÊÊMaintenance: 15-20 mg/kg q24h (assuming no interruption in CRRT,
e.g. line clotting)
Ê UÊ Ìi\Ê >ÞÃÃÊyÜÊÀ>ÌiÃÊÓ°xÊÉ ÊÊVÃÕÌÊ« >À>VÞ
UÊMonitoring:
Ê UÊÊ*>ÌiÌÃÊÜÌ ÊV >}}Ê`>ÞÃÃÊyÜÊÀ>ÌiÃÊÀÊ`>ÞÃÃÊ i`ÊvÀÊ{Ê
hours may need more frequent monitoring (consult pharmacy)
Ê UÊÊ*>ÌiÌÃÊÊÃÌ>LiÊ`>ÞÃÃÊyÜÊÀ>ÌiÃÊÃ Õ`Ê >ÛiÊÌÀÕ} ÊiÛiÊ
checked prior to 4th dose
Peritoneal Dialysis (PD)
UÊInitial dose: 15-20 mg/kg once
UÊÊÊ ÃÕÌÊ« >À>VÞÊvÀÊÀiVi`>ÌÃÊvÀÊÀi`Ã}Ê>`ÊÌÀ}Ê
serum levels.
THERAPEUTIC DRUG MONITORING (LEVELS)
UÊTrough levels are the most accurate and practical method for
monitoring Vancomycin effectiveness and toxicity.
UÊPeak levels should NOT be obtained.
Measuring serum Vancomycin levels
UÊÊ/ÀÕ} ÊiÛiÃÊà Õ`ÊLiÊLÌ>i`ÊÜÌ ÊÎäÊÕÌiÃÊvÊÌ iÊiÝÌÊ`ÃiÊ>ÌÊ
steady-state conditions (approximately before the 4th dose).
UÊÊÊ«>ÌiÌÃÊÜÌ Ê -, ÊÊ i`>ÞÃÃ]ÊÌÊÃÊ«ÀiviÀ>LiÊÌÊLÌ>Ê>Ê
pre-hemodialysis level with the routine laboratory venipuncture on the
morning of hemodialysis. In the event a pre-hemodialysis level is not
obtained, a post-hemodialysis level may be drawn at least six hours
after the dialysis session.
UÊÊ/ÀÕ} ÊiÛiÃÊÃ Õ`ÊLiÊVÃ`iÀi`ÊÊ«>ÌiÌÃÊÜÌ Ê>ÞÊÌ iÊvÜ}Ê
VÀVÕÃÌ>ViÃ\
UÊÊ,iViÛ}Ê>}}ÀiÃÃÛiÊ`Ã}Ê­£xääÊ}Ê+£Ó®ÊÀÊ+nÊÌiÀÛ>
U Serious infections such as meningitis, endocarditis, osteomyelitis,
and MRSA pneumonia.
UÊÊ1ÃÌ>LiÊÀi>ÊvÕVÌÊ­V >}iÊÊ- ÀÊvÊä°xÊ}É`ÊÀÊxä¯ÊvÀÊ
baseline) or dialysis
151
A
B. Vancomycin dosing and monitoring
Ê UÊÊÊ*Ài`>ÞÃÃÊiÛiÊ(preferred)\ÊÓxÊV}ÉÊ­vÀÊi}ÌÃÊVÃ`iÀÊ
Ài`Ã}ÊvÊÎäÊV}É®
Ê UÊ*ÃÌ`>ÞÃÃÊiÛi\ÊÓäÊV}É®
Note:ÊÕÃÌÊÜ>ÌÊÎqÈÊ ÕÀÃÊ>vÌiÀÊÌ iÊi`ÊvÊÌ iÊ`>ÞÃÃÊÌÊ>VVÕÌÊvÀÊ
redistribution of tissue and plasma levels
UÊÊÀÊ«>ÌiÌÃÊÜÌ Ê -, ÊÊ>ÊÃÌ>LiÊ ÊÃV i`Õi]Ê>ÊÀi}iÊÃ Õ`ÊLiÊ
established that coincides with HD (e.g. 500 mg qHD). Once weekly
serum levels can be drawn to monitor for accumulation.
B. Vancomycin dosing and monitoring
A
UÊÊ VÕÀÀiÌÊÌ iÀ>«ÞÊÜÌ Êi« ÀÌÝVÊ>}iÌÃÊ­i°}°Ê>}ÞVÃ`iÃ]Ê
Colistin, Amphotericin B)
UÊ*À}i`ÊVÕÀÃiÃÊ­≥ 5 days) of therapy.
UÊÀiµÕiVÞÊvÊÌÀ}Ê6>VÞVÊÌÀÕ} ÊiÛiÃ\Ê
UÊÊ"ViÜiiÞÊÌÀ}ÊÃÊÀiVi`i`ÊvÀÊ«>ÌiÌÃÊÜÌ ÊÃÌ>LiÊ
renal function who have achieved desired trough levels.
UÊÊÀiÊvÀiµÕiÌÊÌÀ}ÊÃÊÀiVi`i`ÊvÀÊ«>ÌiÌÃÊÜ Ê>ÀiÊ
hemodynamically unstable and/or with changing renal function.
Desired Vancomycin trough levels
UÊÊ*iÕ>]ÊÃÌiÞiÌÃ]Êi`V>À`ÌÃ]ÊL>VÌiÀi>\Ê£xÓäÊV}É
UÊÊ -ÊviVÌÃ\ÊÓäÊV}É
UÊÊ iÕÌÀ«iVÊviÛiÀ]ÊÃÊ>`ÊÃÃÌÀÕVÌÕÀiÊviVÌÃ\Ê£ä£xÊV}É
UÊÊÕÊÃiÀÕÊÌÀÕ} ÊVViÌÀ>ÌÃÊ£äÊV}ÉÊà Õ`Ê>Ü>ÞÃÊ
be maintained to avoid development of resistance.
Monitoring for Toxicity
UÊÊ-iÀÕÊVÀi>ÌiÊÃ Õ`ÊLiÊi>ÃÕÀi`Ê>ÌÊi>ÃÌÊiÛiÀÞÊÌ iÀÊ`>ÞÊÌ>Þ]Ê
then weekly if patient’s renal function remains stable.
UÊÊÌi`Ê`>Ì>ÊÃÕ}}iÃÌÊ>Ê`ÀiVÌÊV>ÕÃ>ÊÀi>ÌÃ «ÊLiÌÜiiÊ
nephrotoxicity and higher serum trough concentrations (>15-20 mcg/
mL). Monitor Vancomycin trough levels (see above for frequency and
indications).
UÊÊÀ>Ê>Õ`}ÞÊÌiÃÌ}ÊÃÊÌÊÀiVi`i`ÊvÀÊ«>ÌiÌÃÊÀiViÛ}Ê
Vancomycin, unless signs and symptoms of ototoxicity became
apparent.
,iviÀiViÃ\
-É-*É- *ÊÕ`iiÃÊÌ iÀ>«iÕÌVÊÌÀ}ÊvÊ6>VÞV\ÊÊÊi>Ì -ÞÃÌÊ
* >À°ÊÓääÆÊÈÈÆÊnÓ°Ê
ÀÃiÊiÌÊ>°ÊÌVÀL>Ê}iÌÃÊ iÌ iÀÊ£nÇÆÊΣ\£ÇÎÇ°
6>`iV>ÃÌiiiÊiÌÊ>°Ê ÊviVÌÊ ÃÊÓ䣣ÆÊxÎ\£Ó{q°
>ÀÌ ÊiÌÊ>°Ê`iÞÊÌÊ£ÈÆÊxä\ÓqÎÈ°
152
153
À>ÊÀi>ÊvÕVÌ\
CBC, BUN, Creatinine
ÊÊÊÊ6>VÞVÊiÛiÊqÊtrough
(see dosing section p. 150)
>ÞÃÃ\
Vancomycin level
(see dosing section p. 150)
At each dialysis session
C. Antimicrobial therapy monitoring
A
Weekly
Weekly, unless change in creatinine
( xä¯ÊvÀÊL>Ãii®]ÊÌ iÊÌÜViÊÜiiÞÊ
,iviÀiVi\Ê*À>VÌViÊÕ`iiÃÊvÀÊ"ÕÌ«>ÌiÌÊ*>ÀiÌiÀ>ÊÌVÀL>Ê/ iÀ>«Þ\Ê ÊviVÌÊ ÃÊÓää{ÆÊÎn\£Èx£°
Vancomycin
UÊÊ}ÊÌiÀÊ`iwi`Ê>ÃÊ≥ 1 week, except for aminoglycosides and Amphotericin B (see below)
UÊÊÀÊÕÃiÊViÊÌ>Ê`Ã}Ê>`ÊÃiÀÕÊiÛiÃÊ >ÛiÊLiiÊiÃÌ>LÃ i`
UÊÊ/ iÃiÊÌÀ}ÊÀiVi`>ÌÃÊ>`ÊÌÀ}ÊvÀÊ>}iÌÃÊÌÊÃÌi`Êà Õ`ÊLiÊ`Û`Õ>âi`]ÊL>Ãi`ÊÊi>V Ê«>Ìi̽ÃÊVV>Êvi>ÌÕÀiÃ]ÊVÕ`}Ê}iiÀ>Ê i>Ì ÊÃÌ>ÌÕÃ]Ê>}i]Ê
underlying conditions and organ dysfunction, concomitant medications, drug treatment history, type of infection, and type and dose of antibiotic
Test
Frequency
Antimicrobial agent(s)
Other
CBC
Weekly
Aminoglycosides (Amikacin, Gentamicin,
Clinical monitoring and patient education
BUN, Creatinine
Twice weekly
Tobramycin, Streptomycin)
for hearing/vestibular dysfunction at
}ÞVÃ`iÊiÛiÊqÊtrough
Weekly
each visit (see p. 149 for vestibular
(see dosing section p. 145)
(twice weekly, if increased risk)
screening method)
BUN, Creatinine, K, Mg, Phos
Twice weekly
Amphotericin B, AmBisome®
CBC, AST, ALT
£qÓÊÜiiÃÊ
CBC, BUN, Creatinine
Weekly
-lactams (Aztreonam, carbapenems,
cephalosporins, penicillins)
add AST/ALT/bilirubin
Weekly
Oxacillin, Nafcillin, carbapenems
add K
Weekly
Penicillin G potassium
AST/ALT/bilirubin
Weekly
Micafungin
BUN, Creatinine
Weekly
Colistin
Clinical monitoring for neurotoxicity
(twice weekly, if increased risk)
(dizziness, paresthesia, vertigo,
confusion, visual disturbances, ataxia)
CBC, BUN, Creatinine , CPK
Weekly
Daptomycin
Clinical monitoring for myopathy
CBC
Weekly
Linezolid
Clinical monitoring for peripheral
neuropathy and optic neuritis
CBC, AST/ALT/bilirubin
Weekly
Rifampin
Drug interactions (monitor start of any
new medications)
CBC, AST/ALT/ bilirubin
£ÊqÊÓÊÜiiÃ
Voriconazole /Posaconazole
Drug interactions (monitor start of any
new medication), visual changes
Recommendations for monitoring patients receiving long-term antimicrobial therapy
°
D. Oral antimicrobial use
A
Oral antimicrobial use in hospitalized patients
When using an agent that is considered to be bioequivalent (no
significant difference in rate and extent of absorption of the therapeutic
ingredient) via the parenteral and oral route, the oral formulation is
preferred if the patient does not have the contraindications listed below.
Contraindications to oral therapy
UÊ *"Ê­VÕ`}Êi`V>ÌîÊ
UÊÊ>LÌÞÊÌÊÌ>iÊÌ iÀÊÀ>Êi`V>ÌÃÊ",ÊÌÊÌiÀ>Ì}Ê>ʵÕ`Ê
diet/tube feeds
UÊi`Þ>VÊÃÌ>LÌÞÊ
UÊ,iViÛ}ÊVÌÕÕÃÊ ÊÃÕVÌ}Ê
UÊÊ-iÛiÀiÊ>ÕÃi>]ÊÛÌ}]Ê`>ÀÀ i>]ÊÊLÃÌÀÕVÌ]Ê`ÞÃÌÌÞ]Ê
mucositis
UÊÊ>>LÃÀ«ÌÊÃÞ`ÀiÊ
U A concomitant disease state that contraindicates the use of oral
medications
NOTE: There are only a limited number of agents that can be
used orally for bacteremia or fungemia; these are noted in
the table below.
Bioavailability of oral antimicrobials
Antimicrobial
% Oral absorption
Should NOT be used orally for bacteremia
ÝVÊ
Ç{ÊqÊä¯
Amoxicillin/Clavulanate (Augmentin®®ÊÊÊ
Ç{ÊqÊä¯
Azithromycin*Ê
ÎnÊqÊnί
i« >iÝÊÊ
ä¯
Cefpodoxime*ÊÊ
{£ÊqÊxä¯
`>ÞVÊÊ
ä¯
ÝÞVÞViÊ
äÊqÊ£ää¯
/iÌÀ>VÞViÊÊ
ÇxÊqÊnä¯
Can be used orally for bacteremia or fungemia
Ciprofloxacin Ê
ÈxÊqÊnx¯
Fluconazole
>ä¯
Linezolid†Ê
£ää¯
iÌÀ`>âiÊ
£ää¯
Moxifloxacin Ê
ä¯
Trimethoprim/sulfamethoxazole†Ê
£ää¯
Voriconazole‡¶Ê
ÈäÊqÊȯ
* Oral absorption is enhanced in presence of food
† Should not be used for S. aureus bacteremia
‡ Oral absorption is decreased in presence of food
¶ Inter-patient variability
ÊÌÊÕÃiÊÜÌ ÊVÌÕÕÃÊÌÕLiÊvii`ÃÊ­6Ê«ÀiviÀÀi`®°Ê*>ÌiÌÃÊÜÌ ÊVÞVVÊÌÕLiÊvii`Ã\Ê
separate oral fluoroquinolone by 2 hours before and 6 hours after tube feeds.
154
Dosing recommendations can vary according to indication and patientspecific parameters. All dosage adjustments are based on creatinine
clearance calculated by Cockcroft-Gault equation.
CrCl =
(140 – age) (weight in kg) x 0.85 (if female)
72 (serum creatinine*)
*
For patients with low muscle, some advocate using a minimum of 1 to avoid
overestimation of CrCl.
†
If patient is on hemodialysis (HD) schedule administration so that patient
receives daily dose immediately AFTER dialysis. For assistance with dosage
adjustments for patients receiving CVVHD or CVVHDF, please call pharmacy.
Drug
Typical dose
(may vary)
CrCl
(mL/min)
Dose adjustment for
renal insufficiency
VÞVÛÀÊ6ÊÊÊ
Ê
Ê
Ê
Acyclovir PO
­iÌ>Ê iÀ«iîÊ
Acyclovir PO
­iÀ«iÃÊ<ÃÌiÀ®ÊÊ
Ê
Amikacin
xq£äÊ}É}Ê+nÊ
Ê
Ê
Ê
200 mg 5x daily
Ê
800 mg 5x daily
Ê
Ê
xäÊ
ÓxqxäÊ
£äqÓ{Ê
†
£äÊÀÊ Ê
>10
£äÊ
>25
£äqÓxÊ
†
£äÊÀÊ
ÝVÊ
Ê
Ê
Amoxicillin
­«iÕ>®Ê
Ê
ÝVÉÊ
V>ÛÕ>>ÌiÊ
Ê
« ÌiÀVÊ Ê
AmBisome®Ê
«VÊ
Ê
Ê
«VÉÊ
ÃÕL>VÌ>Ê
xääq£äääÊ}Ê+£ÓÊ
Ê
Ê
1 g Q8H
Ê
Ê
xääq£äääÊ}Ê+£ÓÊ
Ê
Ê
ä°Çq£Ê}É}Ê+Ó{Ê
ÎqxÊ}É}Ê+Ó{Ê
£qÓÊ}Ê+{qÈÊÊ
Ê
Ê
£°xqÎÊ}Ê+ÈÊ
Ê
Ampicillin/
ÃÕL>VÌ>Ê­vÀÊ
Acinetobacter,
E. faecalis)
âÌ ÀÞVÊ
âÌÀi>ÊÊ
Ê
Ê
iv>âÊ
Ê
Ê
3 g Q4H
Ê
ÎäÊ
£äqÎäÊ
†
£äÊÀÊ Ê
>30
£äqÎäÊ
†
£äÊÀÊ
ÎäÊ
£äqÎäÊ
†
£äÊÀÊ
qÊ
qÊ
xäÊ
£äqxäÊ
†
£äÊÀÊ Ê
≥ÎäÊ
£xqÓÊ
†
≤14 or HD Ê
≥50
£äqxäÊ
†
HD
xq£äÊ}É}Ê+n
xq£äÊ}É}Ê+£Ó
xq£äÊ}É}Ê+Ó{
Ó°xqxÊ}É}Ê+Ó{
200 mg 5x daily
ÓääÊ}Ê+£ÓÊ
800 mg 5x daily
nääÊ}Ê+n
800 mg Q12H
See section on
aminoglycoside dosing
xääq£äääÊ}Ê+£Ó
ÓxäqnÇxÊ}Ê+£Ó
ÓxäqnÇxÊ}Ê+Ó{
1g Q8H
£}Ê+£Ó
1g Q24H
xääq£äääÊ}Ê+£Ó
ÓxäqxääÊ}Ê+£Ó
ÓxäqxääÊ}Ê+Ó{
Ê`Ã>}iÊ>`ÕÃÌiÌ
Ê`Ã>}iÊ>`ÕÃÌiÌ
£qÓÊ}Ê+{qÈ
£qÓÊ}Ê+Èqn
£qÓÊ}Ê+n
£°xqÎÊ}Ê+È
£°xqÎÊ}Ê+£Ó
£°xqÎÊ}Ê+Ó{
3 g Q4H
ÎÊ}Ê+È
3 g Q8H
ÓxäqxääÊ}Ê+Ó{Ê
£qÓÊ}Ê+nÊÊ
Ê
Ê
£qÓÊ}Ê+nÊ
Ê
Ê
qÊ
≥ÎäÊ
£äqÓÊ
†
£äÊÀÊ Ê
≥ÎxÊ
££qÎ{Ê
£äÊÀÊ
†
intermittent HD
†
HD
Ê`Ã>}iÊ>`ÕÃÌiÌ
£qÓÊ}Ê+nÊ
£qÓÊ}Ê+£ÓÊ
£qÓÊ}Ê+Ó{
£qÓÊ}Ê+n
£Ê}Ê+£Ó
£Ê}Ê+Ó{
2 g Q HD, if HD in 2 days
OR 3g Q HD, if HD in 3 days
155
E. Antimicrobial dosing in renal failure insufficiency
A
Antimicrobial dosing in renal insufficiency
E. Antimicrobial dosing in renal failure insufficiency
A
Drug
Typical dose
(may vary)
CrCl
(mL/min)
Dose adjustment for
renal insufficiency
Cefdinir
Ê
300 mg Q12H
Ê
≥30
ÎäÊ
HD†
>60
ÎäqÈäÊ
ÓÊÀÊ
>60
ÎäqÈäÊ
££qÓÊ
££ÊÀÊ
≥ÎäÊ
£äqÓÊ
£äÊÀÊ
≥ÎäÊ
ÎäÊ
HD†Ê
Ê
Ê
Ceftolozane/
Ì>âL>VÌ>Ê
Ê
Ê
600 mg Q12H
Ê
Ê
Ê
600 mg Q8H
Ê
Ê
Ê
£qÓÊ}Ê+nÊ
For PseudomonasÊ
ÓÊ}Ê+nÊ
Ê
1.5 g Q8H
Ê
Ê
Ê
>50
ÎäqxäÊ
£xqÓÊ
£xÊÀÊ
>50
ÎäqxäÊ
£xqÓÊ
£xÊÀÊ
xäÊ
ÎäqxäÊ
£xqÓÊ
£xÊÀÊ
>50
ÎäqxäÊ
£xqÓÊ
ÓÊÀÊ
Ceftolozane/
Ì>âL>VÌ>Ê
­-iÀÕÃÊviVÌîÊ
Ê
3 g Q8H
Ê
Ê
Ê
>50
ÎäqxäÊ
£xqÓÊ
ÉÓÊÀÊ
ivÌÀ>ÝiÊ
ivÌÀ>ÝiÊÊ
(Central nervous
system infections)
Cephalexin
Ê
Ê
Cidofovir
£qÓÊ}Ê+Ó{Ê
ÓÊ}Ê+£ÓÊ
qÊ
qÊ
300 mg Q12H
Î
Ê ääÊ}Ê+Ó{
300 mg QHD
1 g Q8H
Ê£Ê}Ê+£Ó
1 g Q24H
2 g Q8H
£Ê}Ê+nÊ
£Ê}Ê+£Ó
1 g Q24H
£qÓÊ}Ê+£Ó
£qÓÊ}Ê+Ó{
500 mg Q24H
£ääq{ääÊ}Ê+£Ó
£ääq{ääÊ}Ê+Ó{
Ê£ääq{ääÊ}ÊÌ ÀiiÊÌiÃÉ
week
600 mg Q12H
{ääÊ}Ê+£Ó
ÎääÊ}Ê+£Ó
200 mg Q12H
600 mg Q8H
{ääÊ}Ê+n
ÎääÊ}Ê+n
400 mg Q12H
£qÓÊ}Ê+n
£qÓÊ}Ê+£Ó
£qÓÊ}Ê+Ó{
1 g Q24H
1.5 g Q8H
ÇxäÊ}Ê+n
ÎÇxÊ}Ê+n
Load with 750 mg, then
150 mg Q8H
3 g Q8H
£°xÊ}Ê+n
ÇxäÊ}Ê+n
Load with 1.5 g, then
375 mg Q8H
Ê`Ã>}iÊ>`ÕÃÌiÌ
Ê`Ã>}iÊ>`ÕÃÌiÌ
500 mg PO Q6H
Ê
Ê
5 mg/kg Q week for
2 weeks, then every
other week
{ääÊ}Ê+nq£ÓÊÊ
Ê
ÓxäqÇxäÊ}Ê+£ÓÊ
Ê
ÓxäqxääÊ}Ê+£ÓÊ
Ê
*"\ÊÎääÊ}Ê+nÊ
6\ÊÈääÊ}Ê+nÊ
2.5 mg/kg Q12H
Ê
>50
£äqxäÊ
£äÊÀÊ †
≤55 or Cr>1.5
500 mg Q6H
xääÊ}Ê+n
500 mg Q12H
Not recommended
≥ÎäÊ
ÎäÊÀÊ †
≥ÎäÊ
ÎäÊÀÊ †Ê
≥ÎäÊ
ÎäÊ
qÊ
Ê
≥50
ÓäqxäÊ
≤20 or HD†
{ääÊ}Ê+nq£ÓÊ
400 mg Q24H
ÓxäqÇxäÊ}Ê+£Ó
ÓxäqxääÊ}Ê+Ó{
ÓxäqxääÊ}Ê+£Ó
ÓxäqxääÊ}Ê+Ó{
Ê`Ã>}iÊ>`ÕÃÌiÌ
Cefepime
1 g Q8H
Ê
Ê
Ê
Ê
Cefepime
2 g Q8H
­ iÌÀ>ÊiÀÛÕÃÊÊ
Ê
ÃÞÃÌiÊviVÌÃÊÀÊÊ Ê
Pseudomonas®Ê
Ê
ivÌiÌ>Ê
£qÓÊ}Ê+£ÓÊÊ
Ê
Ê
Ê
Ê
iv«`ÝiÊ
£ääq{ääÊ}Ê+£ÓÊ
Ê
Ê
Ceftaroline
Ê
Ê
Ê
Ceftaroline for
,-Ê
Ê
Ê
ivÌ>â`iÊ
«ÀyÝ>VÊ6Ê
Ê
«ÀyÝ>VÊ*"Ê
Ê
>ÀÌ ÀÞVÊ
Ê
`>ÞVÊ
Ê
Colistin
­ ÃÌiÌ >Ìi®Ê
156
†
†
†
†
†
†
†
†
2.5 mg/kg Q12H
Ó°xÊ}É}Ê+Ó{
1.25 mg/kg Q24H
Typical dose
(may vary)
CrCl (mL/min)
Dose adjustment for
renal insufficiency
>«ÌÞVÊÊ
vÀÊi`V>À`ÌÃÉÊ
bacteremia
VÝ>VÊ
ÝÞVÞViÊ
Ertapenem
Ê
Ì >LÕÌÊ
Ê
Èq£äÊ}É}Ê+Ó{ÊÊ
Ê
ÕV>âiÊ
ÓääqnääÊ}Ê+Ó{Ê
≥ÎäÊ
ÎäÊ
HD†Ê
qÊ
qÊ
≥30
ÎäÊÀÊ
≥10
£äÊ
HD†
≥50
Ê
Ê
ÕVÞÌÃiÊ­xq ®Ê
Ê
Ê
Ê
Ganciclovir
­`ÕVÌÊ`Ãi®Ê
Ê
Ê
Ê
Ê
Ê
£Ó°xqÓxÊ}É}Ê+ÈÊ
Ê
Ê
Ê
5 mg/kg Q12H
Ê
Ê
Ê
Ê
xäÊÀÊ †
ÊÊ
{äÊ
Óäq{äÊ
£äq£Ê
£äÊÀÊ †Ê
≥70
xäqÈÊ
Óxq{Ê
£äqÓ{Ê
£äÊÀÊ †
Ganciclovir
­>Ìi>ViÊÊ
`Ãi®Ê
Ê
Ê
5 mg/kg Q24H
Ê
Ê
Ê
Ê
≥70
xäqÈÊ
Óxq{Ê
£äqÓ{Ê
£äÊÀÊ
iÌ>VÊ
qÊ
qÊ
Ã>â`Ê
iâ`Ê
Meropenem
Ê
Ê
Ê
Meropenem
­i}ÌÃ]Ê , ÊÊ
viVÌîÊÊ
Ê
iÌÀ`>âiÊ
V>vÕ}Ê
ÝyÝ>VÊ
Nitrofurantoin
(Macrobid®®Ê
Oseltamivir
­/Ài>ÌiÌ®Ê
Ê
Ê
Oseltamivir
­*À« Þ>ÝîÊ
Ê
Ê
"Ý>VÊ
*iVÊÊÊ
Ê
Ê
ÎääÊ}Ê+Ó{Ê
ÈääÊ}Ê+£ÓÊ
1 g Q8H
Ê
Ê
Ê
2 g Q8H
Ê
Ê
Ê
xääÊ}Ê+nÊ
£ääq£xäÊ}Ê+Ó{Ê
{ääÊ}Ê+Ó{ÊÊ
100 mg Q12H
Ê
75 mg Q12H
Ê
Ê
Ê
75 mg Q24H
Ê
Ê
Ê
£qÓÊ}Ê+{qÈÊÊ
Îq{ÊÊÕÌÃÊ+{Ê
Ê
Ê
qÊ
qÊ
>51
ÓÈqxäÊ
£äqÓxÊ
£äÊÀÊ
>51
ÓÈqxäÊ
£äqÓxÊ
£äÊÀÊ
qÊ
qÊ
qÊ
≥50
xäÊ
>60
ÎäqÈäÊ
£äqÓÊ
£äÊÀÊ
>60
ÎäqÈäÊ
£äqÓÊ
£äÊÀÊ
qÊ
≥xäÊ
£äq{Ê
£äÊÀÊ
Èq£äÊ}É}Ê+Ó{
Èq£äÊ}É}Ê+{n
Èq£äÊ}É}Ê+{n
Ê`Ã>}iÊ>`ÕÃÌiÌ
Ê`Ã>}iÊ>`ÕÃÌiÌ
1 g Q24H
500 mg Q24H
Normal dose Q24H
À>Ê`ÃiÊ+{n
Normal dose QHD session
Normal dose (e.g. 100, 400,
800 mg) Q24H
Load w/normal dose, then
xä¯ÊvÊÀ>Ê`ÃiÊ+Ó{
£Ó°xqÓxÊ}É}Ê+È
£Ó°xqÓxÊ}É}Ê+£Ó
£Ó°xqÓxÊ}É}Ê+Ó{
£Ó°xqÓxÊ}É}Ê+Ó{q{n
5 mg/kg Q12H
Ó°xÊ}É}Ê+£Ó
Ó°xÊ}É}Ê+Ó{
£°ÓxÊ}É}Ê+Ó{
1.25 mg/kg three times/week,
administer after HD
5 mg/kg Q24H
Ó°xÊ}É}Ê+Ó{
£°ÓxÊ}É}Ê+Ó{
ä°ÈÓxÊ}É}Ê+Ó{
0.625 mg/kg three times/
week, administer after HD
Ê iiÊÃiVÌÊÊ>}ÞVÃ`iÊ
dosing
Ê`Ã>}iÊ>`ÕÃÌiÌÊ
Ê`Ã>}iÊ>`ÕÃÌiÌÊ
1 g Q8H
£Ê}Ê+£Ó
xääÊ}Ê+£Ó
500 mg Q24H
2 g Q8H
£Ê}Ê+nÊ
£Ê}Ê+£Ó
1 g Q24H
Ê`Ã>}iÊ>`ÕÃÌiÌ
Ê`Ã>}iÊ>`ÕÃÌiÌ
Ê`Ã>}iÊ>`ÕÃÌiÌ
100 mg Q12H
ÌÊÀiVi`i`
75 mg Q12H
ÇxÊ}Ê+Ó{
ÎäÊ}Ê+Ó{
30 mg QHD session
75 mg Q24H
ÎäÊ}Ê+Ó{
ÎäÊ}Ê+{n
30 mg every other HD session
Ê`Ã>}iÊ>`ÕÃÌiÌ
Îq{ÊÊÕÌÃÊ+{
£°xÊÊÕÌÃÊ+{
1.5 million units Q6H
ÓxäqxääÊ}Ê+ÈÊÊ
£ääÊ}Ê+£ÓÊ
1 g Q24H
Ê
£xqÓxÊ}É}Ê+Ó{ÊÊ
Ê
†
†
†
†
†
†
†
157
E. Antimicrobial dosing in renal failure insufficiency
A
Drug
E. Antimicrobial dosing in renal failure insufficiency
A
Drug
Typical dose
(may vary)
CrCl (mL/min)
Dose adjustment for
renal insufficiency
*«iÀ>VÉÊ
tazobactam
Ê
ΰÎÇxq{°xÊ}Ê+ÈÊ
{äÊ
ÊÊ
Óäq{äÊ
Ê
Ê
ÓäÊÊ
qÊ
Î
Ê °ÎÇxÊ}Ê+ÈÊ­{°xÊ}Ê+È
for Pseudomonas)
Ó
Ê °ÓxÊ}Ê+ÈʭΰÎÇxÊ}Ê+ÈÊvÀÊ
Pseudomonas)
Ó
Ê °ÓxÊ}Ê+nÊ­Ó°ÓxÊ}Ê+ÈÊvÀÊ
Pseudomonas)
2.25 g Q12H (2.25 g Q8H for
Pseudomonas)
Ê`Ã>}iÊ>`ÕÃÌiÌ
≥£äÊ
£äÊ
HD†Ê
qÊ
£xqÎäÊ}É}Ê+Ó{
£ÓqÓäÊ}É}Ê+Ó{
ÓxqÎäÊ}É}Ê+ ÊÃiÃÃ
Ê`Ã>}iÊ>`ÕÃÌiÌ
HD†
*Ã>V>âiÊ
*ÞÀ>â>`iÊ
Ê
+ÕÕ«ÀÃÌÉÊ
dalfopristin
,v>«Ê­/ ®Ê
,v>«Ê
/}iVÞViÊ
/*É-8ÊÊ
­1/ÃÊÀÊViÕÌîÊ
Ê
Ê
/*É-8ÊÊÊ
­* *ÊÀÊÃiÀÕÃÊÊ
systemic infections)
6>>VÞVÛÀÊ
­iÌ>Ê iÀ«iîÊ
Ê
Valacyclovir
­iÀ«iÃÊ<ÃÌiÀ®Ê
Ê
Ê
Valganciclovir
­`ÕVÌÊ`Ãi®Ê
Ê
Ê
Ê
Valganciclovir
­>Ìi>ViÊ`Ãi®Ê
Ê
Ê
Ê
6>VÞVÊ
6ÀV>âiÊ
†
-iiÊ*Ã>V>âiÊ
guidelines p. 18
£xqÎäÊ}É}Ê+Ó{Ê
Ê
Ç°xÊ}É}Ê+nÊÊ
ÈääÊ}Ê+Ó{Ê
ÎääÊ}Ê+nq£ÓÊ
£ääÊ}ÊVi]ÊÌ iÊÊ
50 mg Q12H
*"\Ê£qÓÊ -ÊÌ>LÊ+£ÓÊ
6\Ê£ÈäqÎÓäÊ}Ê+£ÓÊ
­ Ã}ÊÃÊL>Ãi`ÊÊÊ
/*ÊV«iÌ®Ê
xÊ}É}Ê+ÈqnÊ
Ê
xääq£äääÊ}Ê+£ÓÊ
Ê
Ê
1 g Q8H
Ê
Ê
Ê
900 mg Q12H
Ê
Ê
Ê
Ê
900 mg Q24H
Ê
Ê
Ê
Ê
qÊ
-iiÊ6ÀV>âiÊÊ
guidelines p. 19
qÊ
qÊ
qÊ
≥ÎäÊ
Ê
†
ÎäÊÀÊ Ê
Ê
≥ÎäÊ
ÎäÊ
HD†
≥ÎäÊ
£äqÓÊ
£äÊÀÊ †
≥50
Îäq{Ê
£äqÓÊ
£äÊÀÊ †
≥60
{äqxÊ
ÓxqÎÊ
£äqÓ{
£äÊÀÊ †
≥60
{äqxÊ
ÓxqÎÊ
£äqÓ{
£äÊÀÊ †
qÊ
qÊ
Ê`Ã>}iÊ>`ÕÃÌiÌ
Ê`Ã>}iÊ>`ÕÃÌiÌ
Ê`Ã>}iÊ>`ÕÃÌiÌ
£qÓÊ -ÊÌ>LÊ+£ÓÊÀÊ
£ÈäqÎÓäÊ}Ê6Ê+£ÓÊÊ
£qÓÊ -ÊÌ>LÊ+Ó{ÊÀ
£ÈäqÎÓäÊ}Ê6Ê+Ó{
xÊ}É}Ê+ÈqnÊ
Ó°xÊ}É}Ê+Èqn
2.5 mg/kg Q8H
xääq£äääÊ}Ê+£Ó
xääq£äääÊ}Ê+Ó{
500 mg Q24H
1 g Q8H
£Ê}Ê+£Ó
£Ê}Ê+Ó{
500 mg Q24H
900 mg Q12H
{xäÊ}Ê+£Ó
{xäÊ}Ê+Ó{
450 mg Q48H
Not recommended
900 mg Q24H
{xäÊ}Ê+Ó{
{xäÊ}Ê+{n
450 mg twice weekly
Not recommended
Ê-iiÊÃiVÌÊÊÛ>VÞVÊ
dosing
Ê`Ã>}iÊ>`ÕÃÌiÌÊÃ
necessary for PO. IV should
not be administered to patients
with CrCl ≤50 mL/min due to
accumulation of the vehicle.
If patient is on hemodialysis (HD) schedule administration so that patient receives
daily dose immediately AFTER dialysis. For assistance with dosage adjustments for
patients receiving CVVHD or CVVHDF, please call pharmacy.
158
HH
Abdominal infections
Biliary tract infections ..... 39-40
Diverticulitis ......................... 40
Pancreatitis .................... 41-42
Peritonitis, peritoneal
dialysis-related .................. 45
Peritonitis/GI perforation . 42-45
SBP .............................. 42-43
Acute bacterial
rhinosinusitis................... 78-79
Allergy, penicillin ................... 137
Anaerobes......................... 24-25
Amikacin
See Aminoglycosides
Aminoglycosides
Gram-negative infection
dosing ...............................146
Gram-positive synergy
dosing ............................ 148
Mycobacterial infection
dosing ............................ 147
SICU/WICU dosing ............. 148
UTI dosing ......................... 147
Amphotericin B, lipid ............... 16
Antibiotic lock therapy............. 63
Antibiogram....................... 37-38
Antimicrobial dosing
Aminoglycosides
See Aminoglycosides
CNS infections ..................... 73
Renal insufficiency....... 155-158
Surgical prophylaxis .... 121-124
Vancomycin
See Vancomycin
Aspergillosis ......................... 133
Aspiration pneumonia........ 84, 88
Azole drug interactions ...... 21-22
Biliary tract infections......... 39-40
Bloodstream infections
Catheter-related .............. 60-64
Candida ..................117, 134
Enterococcus spp. ............ 62
Gram-negative rods ........... 62
S. aureus.......................... 61
Staph, coagulase-negative . 61
Brain abscess ........................ 76
H H
Candidemia ....................117-118
Candidiasis
Hematologic patient .....134-136
Non-neutropenic host ...115-120
Candiduria ......................115-116
Catheter-related
bloodstream infections.....60-64
Cellulitis..........................100-101
Ceftaroline.................................8
Ceftolozane/tazobactam.........8-9
Central nervous system (CNS)
infections
Antibiotic dosing ...................77
Brain abscess..................76-77
Encephalitis ..........................75
Meningitis ........................73-75
Shunt infection .................76-77
Cholangitis .........................39-40
Cholecystitis .......................39-40
Clostridium difficile
infections.........................47-50
Colistin .................................9-10
Communicable diseases,
reporting ............................140
Community-acquired pneumonia
Empiric therapy ...............83-84
Pathogen-specific therapy . 85-86
COPD exacerbations................82
Cost of antimicrobials .....159-160
Cystic fibrosis.....................91-92
H H
H H
Bacterial vaginosis.................. 57
Daptomycin ....................... 10-11
161
10. Index
A
Index
10. Index
Diarrhea ............................ 51-53
Diabetic foot
infections.................... 103-105
Diverticulitis ............................ 40
Dosing, antimicrobials
See Antimicrobial dosing
H H
Encephalitis ............................ 75
Endocarditis ...................... 65-70
Treatment
Culture-negative ................ 68
Diagnosis .................... 69-70
Fungal ..................... 119-120
Pathogen-specific
therapy ..................... 65-69
Prosthetic valve ........... 68-69
Prophylaxis ........................ 125
Endomyometritis .................... 56
Epidural abscess ........... 108-109
Ertapenem ............................. 11
HH
Febrile neutropenia ........ 129-130
Formulary................................. 7
Fosfomycin ....................... 11-12
Fungal infections
Candida spp
................ 115-120, 134-136
Filamentous fungi ........ 133-134
Prophylaxis, SICU/WICU ..... 120
Fusarium .............................. 133
HH
Gentamicin
See Aminoglycosides
GI perforation ......................... 45
Gonococcal urethritis,
cervicitis, proctitis........... 57-58
Gynecologic infections
Endomyometritis.................. 56
Pelvic inflammatory
disease ............................ 56
162
HH
Healthcare-acquired pneumonia
(not VAP) .........................87-88
H. pylori infection ................54-55
HH
ICD infection ...................... 71-72
ID approval
Antimicrobials ........................ 7
Pager .................................... 6
Infection control............. 139-144
Infectious diarrhea ............. 51-53
Influenza............................ 93-94
Isolation precautions ............. 141
HH
Linezolid.............................12-13
Long-term antimicrobial
therapy...............................153
HH
Meningitis, bacterial ............73-75
Antimicrobial dosing..............77
Empiric therapy ....................73
Pathogen-specific therapy .....74
MDR Gram-negative
organisms .......................28-30
Micafungin..........................17-18
Microbiology.......................31-35
MRSA
Decolonization .............102-103
Soft-tissue infections ....100-101
Surveillance .................142-143
H H
Necrotizing fasciitis ....... 107-108
Neutropenic fever .......... 129-130
Nosocomial pneumonia...... 87-88
H"H
Oncology
Neutropenic fever ........129-130
H*H
P. acnes infection ...............25-26
Pacemaker infection ...........71-72
Pancreatitis ........................41-42
Parasites.................................53
Pelvic inflammatory disease .....56
Penicillin allergy .....................137
Peritonitis/GI perforation .....42-45
Peritoneal dialysis-related ......45
Spontaneous bacterial .....42-43
Post-op / post-procedure
infections ..................105-107
Pneumonia
Community-acquired ........83-84
Healthcare-acquired .........87-88
Ventilator-associated ........88-90
Pneumococcal vaccine ............23
Posaconazole .....................18-19
Pre-operative prophlyaxis.121-124
Price of antimicrobials ....159-160
Prophylactic use of antimicrobials
Endocarditis .......................125
Fluconazole in ICUs .............120
Hematologic
malignancy................ 131-132
Pre-op / pre-procedure 121-124
Solid organ ..................126-128
H,H
Renal insufficiency
Antimicrobial dosing.....155-158
Reported diseases.................140
Resistant Gram-negative
infections.........................28-30
Respiratory viruses .............93-94
Restricted antimicrobials ............7
H-H
SBP ...................................42-43
Sepsis.....................................99
Sexually transmitted
diseases..........................57-59
Shunt infection....................76-77
Sinusitis .............................78-79
Skin, soft-tissue and
bone infections
Cellulitis .......................100-101
Cutaneous abscess .....101-102
Diabetic foot
infection ...................103-105
Necrotizing fasciitis......107-108
Post-op infections ........105-107
Recurrent MRSA ..........102-103
Surgical-site
infections ..................105-107
Vertebral osteomyelitis,
diskitis, epidural
abscess....................108-109
Streptococci ......................24-25
Surgical prophylaxis........121-124
Surgical-site infections ....105-107
Surveillance
CRE ...................................142
MRSA ..........................142-143
VRE ....................................144
Susceptibility testing ...........31-32
Syphilis ..............................58-59
H/H
Therapeutic monitoring
Aminoglycosides..........145-149
Vancomycin .................150-152
Outpatient long-term
antimicrobial therapy ........153
Tigecycline ..............................13
Tobramycin
See Aminoglycosides
Transplant
Antimicrobial prophylaxis
Hematologic
malignancy ............. 131-132
Solid organ................ 126-128
163
10. Index
Oral antimicrobials .................154
Orbital cellulitis ...................80-81
10. Index
Trichomoniasis......................... 57
Trimethoprim/
sulfamethoxazole ..............14-15
Tuberculosis ........................95-98
H1H
Urinary tract infections
Bacterial
Cystitis ........................... 110
Pyelonephritis ................. 111
Urosepsis ....................... 111
Catheter-related .......... 113-114
Fungal ........................ 115-116
H6H
Vancomycin
164
Dosing ....................... 150-152
Monitoring .................. 151-152
Ventilator-associated
pneumonia (VAP) ............. 88-90
Vertebral osteomyelitis, diskitis,
epidural abscess ........ 108-109
Voriconazole ..................... 19-20
VRE Surveillance ................... 144
H7H
Wound infections,
post-op........................105-107
Important Phone Numbers
THE JOHNS HOPKINS HOSPITAL
Antibiotic Approval: . . . . PING “JHH Antibiotic Approval Pager”
Antimicrobial Stewardship Program: . . . . . . . . . . . . . . . . . . . . . . 7-4570
Infectious Diseases Consults: . . . . PING “JHH Infectious Diseases”
Oncology/Transplant Service (Transplant ID) . . . . PING “Transplant/
Oncology Infectious Diseases”
Adult Inpatient Pharmacy (Zayed 7000): . . . . . . . . . . . . . . . . . . . 5-6150
Critical Care and Surgery Pharmacy (Zayed 3121):. . . . . . . . . . . 5-6505
Weinberg Pharmacy: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-8998
Microbiology Lab: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-6510
Hospital Epidemiology & Infection Control: . . . . . . . . . . . . . . . . 5-8384
HEIC Emergency Beeper: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3855
JOHNS HOPKINS BAYVIEW MEDICAL CENTER
Antibiotic Approval: . . . . . . . PING “Bayview Antibiotic Approval”
Infectious Disease Consults:. . PING “Bayview Infectious Diseases”
Bayview Inpatient Pharmacy: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0-0958
Microbiology Lab: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-6510
Hospital Epidemiology & Infection Control: . . . . . . . . . . . . . . . . . 0-0515
The Johns Hopkins Hospital
Antimicrobial Stewardship Program
Intranet: insidehopkinsmedicine.org/amp
Internet: hopkinsmedicine.org/amp
Osler 425
(443) 287-4570 (7-4570)
© Copyright 2015 by The Johns Hopkins Hospital Antimicrobial
Stewardship Program. All rights reserved. No part of this publication
may be reproduced without permission in writing from The Johns
Hopkins Hospital Antimicrobial Stewardship Program.
Cover art: Charlotte Ford Cosgrove, Line Drawing II 33, 2008.