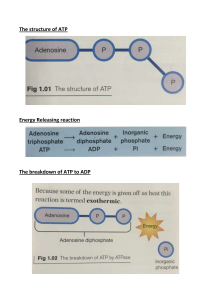
BIOLOGICAL OXIDATION & PRINCIPLE OF ENERGY METABOLISM EDITED & RECOMPOSED BY Dr. Liniyanti D.Oswari MSc. For Medical student, Sriwijaya University Block 8 Citric Acid Cycle Biological oxidation Carbohydrate metabolism Lipid metabolism Glucose, rbc metabolism, glycogen, blood glucose, diabetes Plasma lipoproteins – CM, VLDL, LDL, HDL Protein metabolism Gluconeogenesis Calories Fat contains 9 calories per gram Protein contains 4 calories/gram Carbohydrates has 4 calories per gram (approximately) Anabolism: Building Up ATP produced during catabolism drives anabolism. Excess carbohydrates energy can result in fat synthesis. Humans synthesize 11 of 20 amino acids; remaining 8 essential amino acids must be provided by diet. Anabolism Large complex molecules are synthesized from smaller precursors. Building block molecules (amino acids, sugars and fatty acids) are produced or acquired from the diet. Because anabolic processes include the synthesis of polysaccharides and proteins from sugars and amino acids, the biosynthetic pathways increase order and complexity, they require inputs of free energy (ATP and NADPH). Energy Flows through ATP and redox carriers to couple Catabolic and Anabolic Pathways Nonlinear Metabolic Pathways Metabolism in Muscle Glucose Glycogen Glycogenolysis Ca2+ PKa Lactate BCAA Ile, Val Fatty acids Glycolysis No O2 G6P Pyruvate Krebs cycle b-Oxidation Ca2+ PDH Acetyl-CoA Ca2+ ISDH, aKGDH Production of ATP Electron Transport Chain Eric Niederhoffer Carbohydrate metabolism Glucose Rbc metabolism Glycogen Blood glucose Diabetes Glucose How does the body metabolise glucose? How can we obtain energy from glucose? How is energy derived from glucose? Glucose 2 types of glycolysis: Aerobic g. and anaerobic g. Aerobic g. occurs when oxygen supply is sufficient Anaerobic g. occurs when oxygen supply is lacking In aerobic g.: Oxygen status: sufficient oxygen supply Glucose → pyruvate → TCA → GTP, NADH & FADH2 Substrate-level phosphorylation: GTP → ATP NADH & FADH2 → ETC → ATP: 1 NADH → 3 ATP 1 FADH2 → 2 ATP Anaerobic g.: Oxygen status: insufficient oxygen supply Glucose → pyruvate → lactate Lactate is used via Cori cycle Rbc metabolism What is the source of energy for rbc? Rbc has no mitochondria Rbc depends entirely on glycolysis for ATP Glycogen How is glycogen metabolised by the body? How can we obtain energy from glycogen? How is energy derived from glycogen? Glycogen Glycogen is involved in 2 ways: At high blood glucose level: Glycogen synthesis (glycogenesis) Glycogen breakdown (glycogenolysis) Glycogen is synthesized and stored in liver and muscles At low blood glucose level: Glycogen is broken down (degraded) by enzymes to yield glucose Two enzymes breakdown glycogen to glucose: Branching enzyme Straight chain enzyme Liver vs. muscle glycogen: Body has more liver glycogen than muscle glycogen Liver glycogen is used to maintain blood glucose level Muscle glycogen is used internally Blood glucose What is normal blood glucose level? Note: Blood glucose is determined under fasting condition Plasma is used to determine glucose content Quote values in mmol/L, or mg/dl Normal fasting plasma glucose (FPG) is 4.2-6.2 mmol/L=70 – 110 mg/dl Maintenance of blood glucose Note: There are many factors which regulate blood glucose level Factors: insulin, liver, glucagon, epinephrine, etc When we eat: At high blood glucose level, insulin is secreted Insulin causes cells to take up glucose Cells use glucose for energy When we sleep: The liver maintains blood glucose (by hepatic glycogenolysis) to within acceptable levels between 4.2-6.2 mmol/L =70-110 mg/dl (fasting values) Gluconeogenesis What is gluconeogenesis? Formation of glucose from non-glucose sources such as C-skeletons of glucogenic amino acids Under what conditions does this occur? Gluconeogenesis occurs when blood glucose is low Lipogenesis and Lipolysis Figure 24.14 Protein Metabolism Deaminated amino acids are converted into: Pyruvic acid One of the keto acid intermediates of the Krebs cycle These events occur as transamination, oxidative deamination, and keto acid modification OVERVIEW OF METABOLIC PATHWAYS AND SYSTEMS OF ENERGY METABOLISM Nucleic Acids GLYCOGEN PROTEIN Ribose-5-P Glucose-6-P b b Lactate g Urea e a Glucose f d c TRIACYLGLYCEROLS i j h Amino Acids Free Fatty Acids a l k p Pyruvate Acetyl-CoA m o n Ketone Bodies Figure . Energy systems ATP Energy The meaning of “energy” in energy metabolism In a haste to learn the individual reactions in a pathway, its easy to lose sight of the purpose of the pathway. With energy metabolism, the purpose is to generate energy, generally as ATP or NADH or some high energy compound that will be used in a later anabolic step. Glycolysis and Krebs cycle reactions have a high number of kinase and dehydrogenase enzymes, respectively, for this reason. This class of enzymes is intimately connected with energy production and conservation. Pathways in the cytosol tend to be less energy yielding, whereas those in the mitochondria are almost totally devoted to energy production. This tutorial will bring you closer to understanding why and how cells conserve energy. It will also help you see the logic behind molecular energy calculations. As you listen to your heart pump or move your arm to scratch your head, you should be able to tell what purpose energy serves to life. Hydrolysis Reactions tend to be Strongly Favorable (Spontaneous) Isomerization Reactions Have Smaller Free Energy Changes Complete Oxidation of Reduced Compounds is Strongly Favorable Thermodynamic Laws The First Law of Thermodynamics. A system’s internal energy can change only by the exchange of heat or work with the surroundings. A Statement of Conservation of energy. The Second Law of Thermodynamics. The entropy of an isolated system will tend to increase to a maximum value. The entropy of such a system will not decrease,sucrose will never “ de-diffuse” into corner of the solution. Entropy is a measure of the randomness or disorder in a system. What is energy conservation? The terms energy conservation and energy generation tend to carry the same meaning. Conservation implies “avoiding heat”, or channeling the energy differential between reactants and products into the synthesis of a compound. Because energy as heat cannot be exploited in an isothermal system, biological systems have to conserve energy by biosynthesis. Suppose for example ATP is hydrolyzed during a reaction (click 1). The standard energy differential between reactants and products (Go’) of that reaction is 30.5 kJ/mol. ATP + H2O ADP + PO4 This means the environment of the cell gains 30.5 kJ of heat energy for each mole of ATP hydrolyzed by water. Obviously, this is wasteful. To counter the loss, ATP hydrolysis is coupled with the synthesis of a phosphorylated compound. You saw this as a “coupled” reaction when ATP was needed to produce glucose-6-PO4 or fructose 1,6-bisPO4 (click 1). Glucose + ATP Fructose-6-PO4 + ATP Glucose-6-PO4 + ADP Fructose 1,6-bisPO4 + ADP Now you see that by making glucose-6-PO4 or fructose 1,6-bisPO4, the cell avoids losing the larger part of the ATP hydrolysis energy as heat. This is energy conservation. Click one to go on. LIFE opposes ENTROPY, “S”: 2nd Law of Thermodynamics: a) Entropy & energy: heat exchange-25 oC 25 oC 100 oC 25 oC 25 oC 37 oC 25 oC x 25 oC Direct vs Indirect Energy Production The energy generated in metabolic pathways comes in two forms, direct or indirect. Direct or “substrate level” refers to energy generated during a particular reaction. The production of ATP by reacting ADP with PEP is an example of this type (click 1) COO COO C~OPO3= + ADP C=O CH2 CH3 + ATP Indirect refers to energy channeled into a compound that will return the energy at a later step. High energy compounds such as acyl-phosphates or thioesters fit this example. Another is NADH generated during oxidation reactions in the cytosol or Krebs cycle. When L-malate is oxidzed by NAD+, NADH is generated (click 1). NADH and FADH2 have trapped the electron pair from the oxidation in their structures and will release the energy when they themselves are oxidized. COO COO : C=O HO-C-H + + NAD + NADH + H+ : CH2 CH2 COO COO Calculating energy yield in glycolysis Calculating energy yield helps you see the energy phase of metabolism in real numbers. Take for example the energy yield when glyceraldehyde-3-PO4 is oxidized to pyruvate. How much energy is conserved in this reaction? To determine that number we need to know the pathway. We also need to know if anaerobic or aerobic conditions prevail. First the pathway. There are 5 enzymecatalyzed reactions to consider (click 1). glyceraldehyde-3-PO4 + PO4 + NAD+ 1,3-bisPO4 glycerate + ADP 3-phosphglycerate 2-phosphoglycerate PEP + ADP glyceraldehyde-3-PO4 + PO4 + NAD+ + 2ADP 1,3-bisPO4 glycerate + NADH + H+ 3-phosphoglycerate + ATP 2-phosphoglycerate PEP + H2O pyruvate + ATP + H2O pyruvate + NADH + H+ + 2ATP + 2H2O Removing the common terms on both sides yields a final equation (click 1). We see that the phosphate on glyceraldehyde-3-PO4 and the inorganic PO4 both contribute to formation of ATP. Thus, 2 ATPs are formed by the 5 reactions. Under anaerobic conditions “two” represents the final yield. But, if the reaction was carried out with oxygen and involved the mitochondria, energy to the equivalent of 5 ATPs would result. Click 1 to see why. Energy yield in the mitochondria The mitochondria is the heart of aerobic metabolism. Electrons in NADH and FADH2 are channeled into the electron transport system, which is driven by O2. A large part of energy of oxidation of the electron transport components is preserved in ATP. Each NADH generates the equivalent of 3 ATPs and each FADH2, 2 ATPs for each pair of electrons transferred to oxygen (click 1). O2 NADH : Electron transport H2O NAD ATP ATP ATP NADH from the cytosol yields its electrons indirectly via a shuttle. NADH generated by the 3 NAD-linked dehydrogenases in the Krebs cycle provide most of the energy. For example, each citrate molecule oxidized to CO2 and H2O generates the equivalent of 36 ATPs. Click 1 to see how this value was obtained. Energy yield in the Krebs cycle A cycle implies the last intermediate returns to the front. Each turn of the Krebs cycle results in the loss of 2 carbons as CO2 and generates 3NADH, one FADH2 and one GTP (click 1). A 2-carbon compound, such as the acetate group on acetyl-CoA, therefore, yields 12 ATPs of energy. Acetyl-CoA citrate oxaloacetate isocitrate CO2 NADH NADH a-ketoglutarate malate NADH fumarate CO2 succinyl-CoA FADH2 GTP succinate C4H4O5 + 31/2 O2 C6H8O7 + 41/2O2 C4H6O5 + 5 O2 4CO2 + 2H2O 6CO2 + 4H2O 4CO2 + 3H2O Now, suppose instead of acetylCoA we want to determine the ATP yield when oxaloacetate (OAA) is oxidized (click 1). First write the equation for the oxidation (click 1). OAA yields 4 moles of CO2 for each mole oxidized. Thus, 2 turns of the cycle are needed to oxidize all of the carbons in OAA to CO2. Two turns is equivalent to 24 ATPs. Performing the same analysis for citrate shows 6CO2 liberated, or 3 turns of the cycle (click 1). Thus, citrate yield 36 ATPs, or one third more energy than OAA. Finally lets consider the oxidation of malate (click 1). Malate has 4 carbons, which means the oxidation will generate 4CO2. But, we also need to oxidize malate to OAA, which generates one NADH. Thus 3 more ATPs than OAA, i.e., 24 + 3= 27 ATPs. Click 1 to test and expand your understanding. Thermodynamics and Metabolism A. Free-Energy Change • Free-energy change (G) is a measure of the chemical energy available from a reaction G = Gproducts - Greactants • H = change in enthalpy • S = change in entropy Relationship between energy and entropy • Both entropy and enthalpy contribute to G G = H - TS (T = degrees Kelvin) -G = a spontaneous reaction in the direction written +G = the reaction is not spontaneous G = 0 the reaction is at equilibrium The Standard State (Go) Conditions • Reaction free-energy depends upon conditions • Standard state (Go) - defined reference conditions Standard Temperature = 298K (25oC) Standard Pressure = 1 atmosphere Standard Solute Concentration = 1.0M • Biological standard state = Go’ Standard H+ concentration = 10-7 (pH = 7.0) rather than 1.0M (pH = 1.0) B. Equilibrium Constants and Standard Free-Energy Change • For the reaction: A + B C+D Greaction = Go’reaction + RT ln([C][D]/[A][B]) • At equilibrium: Keq = [C][D]/[A][B] and Greaction = 0, so that: Go’reaction = -RT ln Keq C. Actual Free-Energy Change Determines Spontaneity of Cellular Reactions • When a reaction is not at equilibrium, the actual free energy change (G) depends upon the ratio of products to substrates • Q = the mass action ratio G = Go’ + RT ln Q Where Q = [C]’[D]’ / [A]’[B]’ The Free Energy of ATP • Energy from oxidation of metabolic fuels is largely recovered in the form of ATP Go' = - RT ln K'eq Variation of equilibrium constant with Go‘ (25 oC) K'eq G º' kJ/mol Starting with 1 M reactants & products, the reaction: 10 4 - 23 proceeds forward (spontaneous) 10 2 - 11 proceeds forward (spontaneous) 100 = 1 10 10 0 is at equilibrium -2 + 11 reverses to form “reactants” -4 + 23 reverses to form “reactants” Energy coupling A spontaneous reaction may drive a non-spontaneous reaction. Free energy changes of coupled reactions are additive. A. Some enzyme-catalyzed reactions are interpretable as two coupled half-reactions, one spontaneous and the other non-spontaneous. At the enzyme active site, the coupled reaction is kinetically facilitated, while individual half-reactions are prevented. Free energy changes of half reactions may be summed, to yield the free energy of the coupled reaction. For example, in the reaction catalyzed by the Glycolysis enzyme Hexokinase, the half-reactions are: ATP + H2O ADP + Pi Go' = -31 kJ/mol Pi + glucose glucose-6-P + H2O Go' = +14 kJ/mol Coupled reaction: ATP + glucose ADP + glucose-6-P Go' = -17 kJ/mol The structure of the enzyme active site, from which H2O is excluded, prevents the individual hydrolytic reactions, while favoring the coupled reaction. B. Two separate reactions, occurring in the same cellular compartment, one spontaneous and the other not, may be coupled by a common intermediate (reactant or product). A hypothetical, but typical, example involving PPi: Enzyme 1: A + ATP B + AMP + PPi Go' = + 15 kJ/mol Enzyme 2: PPi + H2O 2 Pi Go' = – 33 kJ/mol Overall spontaneous reaction: Go' = – 18 kJ/mol A + ATP + H2O B + AMP + 2 Pi Pyrophosphate (PPi) is often the product of a reaction that needs a driving force. Its spontaneous hydrolysis, catalyzed by Pyrophosphatase enzyme, drives the reaction for which PPi is a product. “High energy” bonds: Compounds with “high energy bonds” are said to have high group transfer potential. For example, Pi may be spontaneously cleaved from ATP for transfer to another compound (e.g., to a hydroxyl group on glucose). Potentially, 2 ~P bonds can be cleaved, as 2 phosphates are released by hydrolysis from ATP. AMP~P~P AMP~P + Pi AMP~P AMP + Pi (ATP ADP + Pi) (ADP AMP + Pi) Alternatively: AMP~P~P AMP + P~P P~P 2 Pi (ATP AMP + PPi) (PPi 2Pi) ATP often serves as an energy source. Hydrolytic cleavage of one or both of the "high energy" bonds of ATP is coupled to an energy-requiring (nonspontaneous) reaction. AMP functions as an energy sensor & regulator of metabolism. When ATP production does not keep up with needs, a higher portion of a cell's adenine nucleotide pool is AMP. AMP stimulates metabolic pathways that produce ATP. • Some examples of this role involve direct allosteric activation of pathway enzymes by AMP. • Some regulatory effects of AMP are mediated by the enzyme AMP-Activated Protein Kinase. A reaction important for equilibrating ~P among adenine nucleotides within a cell is that catalyzed by Adenylate Kinase: ATP + AMP 2 ADP The Adenylate Kinase reaction is also important because the substrate for ATP synthesis, e.g., by mitochondrial ATP Synthase, is ADP, while some cellular reactions dephosphorylate ATP all the way to AMP. The enzyme Nucleoside Diphosphate Kinase (NuDiKi) equilibrates ~P among the various nucleotides that are needed, e.g., for synthesis of DNA & RNA. NuDiKi catalyzes reversible reactions such as: ATP + GDP ADP + GTP, ATP + UDP ADP + UTP, etc. Inorganic polyphosphate Many organisms store energy as inorganic polyphosphate, a chain of many phosphate residues linked by phosphoanhydride bonds: P~P~P~P~P... Hydrolysis of Pi residues from polyphosphate may be coupled to energy-dependent reactions. Depending on the organism or cell type, inorganic polyphosphate may have additional functions. E.g., it may serve as a reservoir for Pi, a chelator of metal ions, a buffer, or a regulator. Why do phosphoanhydride linkages have a high G of hydrolysis? Contributing factors for ATP & PPi include: Resonance stabilization of products of hydrolysis exceeds resonance stabilization of the compound itself. Electrostatic repulsion between negatively charged phosphate oxygen atoms favors separation of the phosphates. Phosphocreatine (creatine phosphate), another compound with a "high energy" phosphate linkage, is used in nerve & muscle for storage of ~P bonds. O - O CH3 H N P O - C N O CH2 C NH2+ phosphocreatine Creatine Kinase catalyzes: Phosphocreatine + ADP ATP + creatine This is a reversible reaction, though the equilibrium constant slightly favors phosphocreatine formation. Phosphocreatine is produced when ATP levels are high. When ATP is depleted during exercise in muscle, phosphate is transferred from phosphocreatine to ADP, to replenish ATP. O- O- O C C CH2 PEP O- O ADP ATP OPO32H+ C C C O- O OH CH2 enolpyruvate C O CH3 pyruvate Phosphoenolpyruvate (PEP), involved in ATP synthesis in Glycolysis, has a very high G of Pi hydrolysis. Removal of Pi from ester linkage in PEP is spontaneous because the enol spontaneously converts to a ketone. The ester linkage in PEP is an exception. NH2 N N ester linkage O -O P O- O O P O- N O O P O CH2 O- ATP (adenosine triphosphate) adenine O H H OH H OH H N ribose Generally phosphate esters, formed by splitting out water between a phosphoric acid and an OH group, have a low but negative G of hydrolysis. Examples: the linkage between the first phosphate and the ribose hydroxyl of ATP. O 6 CH 2 4 OH P OH O 5 H O H OH 3 H OH CH2 H CH O 1 H 2 HO OH OH OH glucose-6-phosphate CH2 O P O- O- glycerol-3-phosphate Other examples of phosphate esters with low but negative G of hydrolysis: the linkage between phosphate & a hydroxyl group in glucose-6-phosphate or glycerol-3phosphate. O Protein Kinase OH + ATP Protein Protein O P O- + ADP OPi H2O Protein Phosphatase the linkage between phosphate and the hydroxyl group of an amino acid residue in a protein (serine, threonine or tyrosine). Regulation of proteins by phosphorylation and dephosphorylation will be discussed later. ATP has special roles in energy coupling & Pi transfer. G of phosphate hydrolysis from ATP is intermediate among examples below. ATP can thus act as a Pi donor, & ATP can be synthesized by Pi transfer, e.g., from PEP. Compound Go' of phosphate hydrolysis, kJ/mol Phosphoenolpyruvate (PEP) - Phosphocreatine - Pyrophosphate - ATP (to ADP) - Glucose-6-phosphate - Glycerol-3-phosphate - Kinetics vs Thermodynamics: A high activation energy barrier usually causes hydrolysis of a “high energy” bond to be very slow in the absence of an enzyme catalyst. This kinetic stability is essential to the role of ATP and other compounds with ~ bonds. If ATP would rapidly hydrolyze in the absence of a catalyst, it could not serve its important roles in energy metabolism and phosphate transfer. Phosphate is removed from ATP only when the reaction is coupled via enzyme catalysis to some other reaction useful to the cell, such as transport of an ion, phosphorylation of glucose, or regulation of an enzyme by phosphorylation of a serine residue. Pathway Eukaryote Prokaryote Glycolysis Cytoplasm Cytoplasm Intermediate step Cytoplasm Cytoplasm Krebs cycle Mitochondrial matrix Cytoplasm ETC Mitochondrial inner Plasma membrane membrane ATP produced from complete oxidation of 1 glucose using aerobic respiration Glycolysis Intermediate step Krebs cycle 2 By oxidative phosphorylation From From NADH FADH (2X3)=6 0 0 (2X3)=6 2 (6X3)=18 (2X2)=4 Total 4 30 4 Pathway By substratelevel phosphorylation 36 ATPs are produced in eukaryotes. Oxidation-Reduction Oxidation: the loss of electrons Reduction: the gain of electrons Oxidation-reduction (redox) reaction: any reaction in which electrons are transferred from one species to another Ared + Box Aox + Bred Look for ions that change in charge i.e. Zn (s) + Cu2+ Zn2+ (aq) + Cu (s) The Zn lost electrons ….. It was oxidized So the Cu was reduced! Oxidation-Reduction Remember: “LEO the lion says GER” Loss Electrons OXIDATION Gain Electrons REDUCTION Oxidation-Reduction +4 +3 +2 +1 0 -1 -2 -3 -4 You Try It Oxidized Fe2+ Fe 3+ Reduced OXIDIZED! Cu 2+ Cu (s) Hint: Cu (s) is Cu 0 REDUCED! Oxidation-Reduction Example: if we put a piece of zinc metal in a beaker containing a solution of copper(II) sulfate some of the zinc metal dissolves some of the copper ions deposit on the zinc metal the blue color of Cu2+ ions gradually disappears In this oxidation-reduction reaction zinc metal loses electrons to copper ions Zn(s) 2+ Zn (aq) + 2 e Zn is oxidized copper ions gain electrons from the zinc 2+ Cu ( aq) + 2 e - Cu( s) Cu 2+ is red uced Oxidation-Reduction we summarize these oxidation-reduction relationships in this way electrons flow from Zn to Cu2 + Cu2 + (aq) loses electrons ; gains electrons ; is red uced is oxidized gives electrons tak es electrons to Cu 2+ ; is th e from Zn; is th e red ucing agent oxidizin g agent Zn(s) + 2+ Zn ( aq) + Cu( s) Oxidation-Reduction using these alternative definitions for the combustion of methane electrons are transferred from carbon to oxygen CH4 (g) gains O and loses H; is oxidized + O2 (g) gains H; is reduced is the reducing is the oxidizing agent agent CO2 (g) + H2 O(g) Oxidation-Reduction Five important types of redox reactions combustion: burning in air. The products of complete combustion of carbon compounds are CO2 and H2O. respiration: the process by which living organisms use O2 to oxidize carbon-containing compounds to produce CO2 and H2O. The importance of these reaction is not the CO2 produced, but the energy released. rusting: the oxidation of iron to a mixture of iron oxides 4Fe(s) + 3O2 ( g) 2Fe2 O3 ( s) bleaching: the oxidation of colored compounds to products which are colorless batteries: in most cases, the reaction taking place in a battery is a redox-reaction Oxidation-Reduction Reactions Reduced organic compounds serve as fuels from which electrons can be stripped off during oxidation Reversible Oxidation of a Secondary Alcohol to a Ketone Many biochemical oxidation-reduction reactions involve transfer of two electrons In order to keep charges in balance, proton transfer often accompanies electron transfer In many dehydrogenases, the reaction proceeds by a stepwise transfers of proton ( H+ ) and hydride ( :H- ) NAD and NADP are Common Redox Cofactors These are commonly called pyridine nucleotides They can dissociate from the enzyme after the reaction In a typical biological oxidation reaction, hydride from an alcohol is transferred to NAD+ giving NADH Heat of Reaction In almost all chemical reactions, heat is either given off or absorbed example: the combustion (oxidation) of carbon liberates 94.0 kcal per mole of carbon oxidized C(s) + O2 (g) CO2 (g) + 94.0 kcal Heat of reaction: the heat given off or absorbed in a chemical reaction exothermic reaction: one that gives off heat – feels hot endothermic reaction: one that absorbs heat – feels cold Heat of Reaction So, is this reaction exothermic or endothermic? C(s) + O2 (g) CO2 (g) + 94.0 kcal Heat is released (on the product side) It’s Exothermic How about: 2 NH3 + 22.0 kcal N2 (g) + 3 H2 (g) Heat enters with reactant (on the reactant side) It’s Endothermic What are Functions of NAD, NADP, FAD? Electron carriers Oxidation / reduction reactions NAD and catabolic reactions -- substrate oxidation -- H- used for ATP synthesis NADP and anabolic reactions -- substrate reduction -- e.g., --COOH to C=O to C-OH Using NADH to make ATP Electron transport and oxidative phosphorylation You should be able to complete a table to calculate energy yields from glucose or fatty acids (of any given length) -- assuming 2.5 ATP per NADH (1.5 per glycolytic NADH) and 1.5 ATP per FADH From glucose Glycolysis ____ NADH x ____ ATP ____ ATP Transition Rx ____ NADH x ____ ATP x 2 pyr Krebs cycle ____ NADH x ____ ATP x 2 pyr ____ FADH x ____ ATP x 2 pyr ____ GTP x ____ ATP x 2 pyr Total ATP yield _______ _______ _______ _______ _______ _______ 30 From a 12 carbon fatty acid B-oxidation ____ FADH x ____ ATP x 6 acCoA ____ NADH x ____ ATP x 6 acCoA Krebs cycle ____ NADH x ____ ATP x 6 acCoA ____ FADH x ____ ATP x 6 acCoA ____ GTP x ____ ATP x 6 acCoA Total ATP yield _______ _______ _______ _______ _______ 84 What are the biosynthetic roles Of these pathways? Table . Summary of redox complexes of the electron transport chain Complex designation Electron transport function Complex I (NADH-Q reductase) Iron containing flavoprotein oxidizes NADH to NAD+; transfers electrons to coenzyme Q Complex II (Succinate-Q reductase) FAD prosthetic group; SDH oxidizes succinate to fumarate; electron transfer to CoQ Complex III (cytochrome reductase) Heme-iron cytochromes reduces cytochrome C electron transfer CoQ to cyt C Complex IV (cytochrome oxidase) Copper and iron containing heme oxidizes cytochrome C; reduces ½O2 to H2O electron transfer cyt C to O2 CLINICAL CORRELATE – RESPIRATORY CHAIN DEFECT defects in each complex of the respiratory chain have been identified associated with lactic acidemia because the high NADH concentration favors the formation of lactate from pyruvate blood lactate may be elevated 30-fold blood pyruvate increases up to 10-fold NADH inhibition of TCA cycle + PDH pyruvate pyruvate + NADH Lactate • ketones produced due to inhibition of TCA cycle and blood ratio of b-hydroxybutyrate to acetoacetate increased in response to increased mitochondrial NADH to NAD ratio • serum alanine is also increased due to decreased pyruvate metabolism e- = electrons CYTOPLASM O2 OUTER MEMBRANE eCoQ Glucose NAD+ e- GLYCOLYSIS FADH2 NADH eNAD+ Pyruvate Dihydroxyacetone phosphate (DHAP) (1) Glycerol e3-phosphate INNER MEMBRANE MATRIX e- DHAP (2) G3P Glycerol-3-phosphate dehydrogenase FAD Glycerol phosphate shuttle. Cytoplasmic glycerol 3-phosphate dehydrogenase (1) oxidizes NADH. Glycerol 3-phosphate dehydrogenase in the inner membrane (2) reduces FAD to FADH2. Glucose NAD+ e- = electrons GLYCOLYSIS INNER OUTER MEMBRANE MEMBRANE e- e- Pyruvate NADH e- OAA (6) Asp NADH Glu Glu (5) eAsp (4) (3) (1) NAD+ OAA Complex I eMalate KG KG (2) CYTOPLASM The malate-aspartate shuttle. eMalate NAD+ MATRIX Mitochondrion has two membrane bilayers Inner mitochondria membrane Matrix Cytochrome B, Cytochrome C, Fe-S proteins, etc. Electron Transport Chain ATP Synthase NADH + ATP production NAD Matrix e.g. in brown fats for heat generation in small mammals. Oxidative Phosphorylation takes place in mitochondria for more ATP production • Glycolysis takes place in the cytoplasm; after glycolysis, pyruvate is added with CoA using NAD+ to become Acetyl CoA, CO2 and NADH. • Acetyl CoA is the fuel for Krebs Cycle to take place in the matrix. • Oxidative phosphorylation depends on electron transfer and the respiratory chain linking to TCA cycle create proton gradient across the inner membrane of mitochondria. • The proton gradient powers the synthesis of ATP using ATP Synthase • When these steps are blocked or uncoupled by uncoupling proteins, no ATP made but only heat energy produced. Summary In this chapter, we learned that the rules of thermodynamics, and organic chemistry still apply to living systems. For example: • Group transfer reactions are favorable when the free energy of products is much lower than the free energy of reactants. In biochemical phosphoryl transfer reactions, the good phosphate donors are destabilized by electrostatic repulsion, and the reaction products are often stabilized by resonance. • Unfavorable reactions can be made possible by chemically coupling a highly favorable reaction to the unfavorable reaction. For example, ATP can be synthesized in the cell using energy in phosphoenolpyruvate. • Oxidation-reduction reaction commonly involve transfer of electrons from reduced organic compounds to specialized redox cofactors. The reduced cofactors can be used in the biosynthesis, or may serve as a source of energy for ATP synthesis. Review Questions • How does muscle produce ATP (carbohydrates, fatty acids, branched-chain amino acids)? • What are the key Ca2+ regulated steps? Test and Expand your understanding about energy 1. How many phosphorylated intermediates are in the Krebs cycle? Ans: None. GTP is synthesized from GDP + Pi. GTP, however, is not a cycle intermediate. 2. How is ATP generated in the Krebs cycle? Ans: Indirectly. The reduced coenzymes, NADH and FADH2 shuttle electrons to the electron transport system and energy is preserved by ATP synthesis 3. Is pyruvate → acetyl-CoA the only way to enter carbons into the Krebs cycle? Ans: No. Any compound that can be converted into a Krebs cycle intermediate will contribute carbons to the Krebs cycle. This applies to aspartate and glutamate, which form OAA and a-ketoglutarate, respectively. 4. What numbers should I remember in order to calculate energy yield in the Krebs cycle? Ans: In terms of ATP, remember that each NADH is equivalent to 3, each FADH2 to 2, and each turn of the cycle 12 ATPs. 5. How many ATPs are generated when succinyl-CoA is oxidized in the cycle? Ans: 30. One for GTP, two for FADH2 and 3 for NADH must be added to the 24 for 2 turns of the cycle. Kebutuhan energi Manusia • Gambaran Kkal/hari • • • • • • Energi digunakan Wanita dewasa normal Laki-laki dewasa normal Pasien Bed rest Bayi baru lahir Remaja perempuan aktif Remaja pria aktif 700 – 2000 2400 – 2800 1300 – 1800 350 – 450 2400 – 2600 3100 - 3600 Energi yang digunakan • • • • • • Aktifitas Kkal/mnt Duduk sambil istirahat 0.7 – 2.0 Berjalan 2.0 – 6.0 Lari cepat 15 atau lebih Lari jarak jauh/Maraton 10 atau lebih Balap sepeda 10 atau lebih Total Energy Requirement (Adults)(TERA) • Energy Allowance Based on Activity Level TERA = IBW (k) x Physical Activity Activity level: Bed rest (hospital patients) 27.5 Sedentary (mostly sitting) 30.0 Light (Tailor, Nurse, Physician, Jeepney driver) 35.0 Moderate (Carpenter, Painter, Heavy housework) 40.0 Very active (swimming, lumberman) 45.0 Rumus Harris Bennedict • • • • Biasanya menghitung BMR melalui rumus sbb: BEE = Basal Energy expenditure Harris Bennedict BEE female: 655 + 9.7( W kg)+1.85 (Ht cm)– 4.7(A) • BEE male: 66.5 + 13.75 (W kg) + 5(Hg cm)–6.8 (A) • Total Energy: BEE + Physical activity + TEF Faktor yang mempengaruhi BMR(Basal Metabolic Rate) • Meningkat: – Pertumbuhan – Badan Kurus & Tinggi – Laki-laki > Perempuan – Demam , stress – Kehamilan /menyusui. – Meningkat pada thyroxin ( Thyrotoxocosis Thermal Effect of Food • TEF = Thermal effect of food • Meningkat energi yang digunakan setelah makan.. • 5-10% of BMR • Digunakan untuk mencerna, pencernaan dan asimilasi dari Makanan Yang dimakan • Contoh: 5% x 1320 = 60 Cal Total Energy(TE) • TE = BMR +TEF + Activities • Aktifitas: Apa saja kegiatan rutin – Sedentary 25-35% BMR – Light 35-50% – Moderate 50-70% – Heavy >70% – http://www.americaonthemove.org/ – USATODAY.com - Study: Obesity rises faster in poor teens