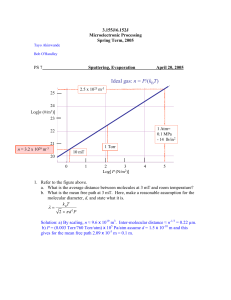
Materials Synthesis techniques Thin Films By Dr. Hafiz Naeem-ur-Rehman Content Physical vapor deposition (PVD) – Thermal evaporation – Sputtering – Evaporation and sputtering compared – MBE – Laser sputtering – Ion Plating – Cluster-Beam Chemical vapor deposition (CVD) – Reaction mechanisms – Step coverage – CVD overview Epitaxy Electrochemical Deposition Physical Vapor Deposition (PVD) PVD Physical methods produce the atoms that deposit on the substrate – Evaporation – Sputtering Sometimes called vacuum deposition because the process is usually done in an evacuated chamber PVD is mostly used for metals. Dielectrics can be deposited using specialized equipment Evaporation Rely on thermal energy supplied to the crucible or boat to evaporate atoms Evaporated atoms travel through the evacuated space between the source and the sample and stick to the sample – Few, if any, chemical reactions occur due to low pressure – Can force a reaction by flowing a gas near the crucible Surface reactions usually occur very rapidly and there is very little rearrangement of the surface atoms after sticking – Thickness uniformity and shadowing by surface topography, and step coverage are issues Evaporation The number of molecules leaving a unit area of evaporant per second N = No exp- e kT Physical vapor deposition (PVD): thermal evaporation N (molecules/unit area/unit time) = 3. 513. 10 22Pv(T)/ (MT) 1/2 This is the relation between vapor pressure of the evaporant and the evaporation rate. If a high vacuum is established, most molecules/atoms will reach the substrate without intervening collisions. Atoms and molecules flow through the orifice in a single straight track, or we have free molecular flow : Kn = /D > 1 The fraction of particles scattered by collisions with atoms of residual gas is proportional to: The source-to-wafer distance must be smaller than the mean free path (e.g, 25 to 70 cm) The cosine law A ~ cos cos /d2 http://www.ee.byu.edu/cleanroom/metal.parts/vaporpressure.jpg Mean Free Path ~ 63% of molecules undergo a collision in a distance less than and ~0.6% travel more than 5 . 1 2d o2 n PV nRT (Ideal Gas Law) 0.05 P(in torr ) – where do is the diameter of the evaporatant and n is the concentration of gas molecules in the chamber Evaporation The vacuum is usually < 10-5 torr – 4x10-6 torr, = 18 inches The source heater can be – Resistance (W, Mo, Ta filament) Contaminants in filament systems are Na or K because they are used in the production of W – E-beam (graphite or W crucible) E-beam is often cleaner although S is a common contaminant in graphite – Top surface of metal is melted during evaporation so there is little contamination from the crucible More materials can be evaporated (high melting-point materials) A downside of e-beam is that X-rays are produced when the electron beam hits the Al melt – These X-rays can create trapped charges in the gate oxide – This damage must be removed by annealing Heat Sources Resistance e-beam RF Laser Advantages No radiation Low contamination No radiation No radiation, low contamination Disadvantages Contamination Radiation Contamination Expensive Crucibles used for evaporation Thermal Evaporation http://www.lesker.com/newweb /Deposition_Sources/ThermalEv aporationSources_Resistive.cfm E-beam Evaporation http://www.fen.bilkent.edu.tr/~aykutlu/msn551/evaporation.pdf PVD Evaporation Evaporating alloys is difficult Because of the differing vapor pressures. – Composition of the deposited material may very different from that of the target material The problem can be overcome by – Using multiple e-beams on multiple sources This technique causes difficulties in sample uniformity because of the spacing of the sources – Evaporating source to completion (until no material is left) Dangerous to do in e-beam system Evaporation Compounds are also hard to evaporate because the molecular species may be different from the compound composition – Energy provided may be used to dissociate compound. – When evaporating SiO2, SiO is deposited. Evaporation in a reactive environment (flowing O2 gas near crucible during deposition) helps reconstitute oxide. Evaporation Advantages – Little damage to the wafer – Deposited films are usually very pure Disadvantages – Materials with low vapor pressures are very difficult to evaporated Refractory metals High temperature dielectrics – No in situ precleaning – Limited step coverage – Film adhesion can be problematic Other PVD Techniques Other deposition techniques include – Sputter deposition (DC, RF, and reactive) – Bias sputtering – Magnetron sputtering – Collimated and ionized sputter deposition – Hot sputter deposition Sputtering Sputter deposition is done in a vacuum chamber (~10mTorr) as follows: – Plasma is generated by applying an RF signal producing energetic ions. – Target is bombarded by these ions (usually Ar+). – Ions knock the atoms from the target. – Sputtered atoms are transported to the substrate where deposition occurs. Sputtering Wide variety of materials can be deposited because material is put into the vapor phase by a mechanical rather than a chemical or thermal process (including alloys and insulators). Excellent step coverage of the sharp topologies because of a higher chamber pressure, causing large number of scattering events as target material travels towards wafers. Film stress can be controlled to some degree by the chamber pressure and RF power. http://www.knovel.com Deposition conditions Temperature: Room to higher Pressure: 100mtorr – compromise between increasing number of Ar ions and increasing scattering of Ar ions with neutral Ar atoms Power – Heating of target material Low temperature metals can melt from temperature rise caused by energy transfer from Ar ions Sputter sources Magnetron – Magnetic field traps freed electron near target – Move in helical pattern, causing large number of scattering events with Ar gas – creating high density of ionized Ar Ion beam – Plasma of ions generated away from target and then accelerated toward start by electric field Reactive sputtering – Gas used in plasma reacts with target material to form compond that is deposited on wafer Ion-assisted deposition – Wafer is biased so that some Ar ion impact its surface, density the deposited film. May sputter material off of wafer prior to deposition for in-situ cleaning. Sputtering Advantages – Large-size targets, simplifying the deposition of thins with uniform thickness over large wafers – Film thickness is easily controlled by fixing the operating parameters and simply adjusting the deposition time – Control of the alloy composition, step coverage, grain structure is easier obtained through evaporation – Sputter-cleaning of the substrate in vacuum prior to film deposition – Device damage from X-rays generated by electron beam evaporation is avoided. Disadvantages – High capital expenses are required – Rates of deposition of some materials (such as SiO2) are relatively low – Some materials such as organic solids are easily degraded by ionic bombardment – Greater probability to introduce impurities in the substrate because the former operates under a higher pressure Pulsed Laser Deposition (PLD) Physical vapor deposition (PVD): Laser Ablation Laser sputter deposition – Complex compounds – High Temperature Super conductors, – biocompatible – ceramics Thin Film Deposition Transfer atoms from a target to a vapor (or plasma) to a substrate Thin Film Deposition Transfer atoms from a target to a vapor (or plasma) to a substrate After an atom is on surface, it diffuses according to: D=Doexp(-eD/kT) eD is the activation energy for diffusion ~ 2-3 eV kT is energy of atomic species. Want sufficient diffusion for atoms to find best sites. Either use energetic atoms, or heat the substrate. Pulsed Laser Deposition CCD /PMT spectrometer laser beam Substrates Target Pulsed Laser Deposition CCD /PMT spectrometer laser beam Substrates Target Target: Just about anything! (metals, semiconductors…) Laser: Typically excimer (UV, 10 nanosecond pulses) Vacuum: Atmospheres to ultrahigh vacuum Advantages of PLD Flexible, easy to implement Growth in any environment Exact transfer of complicated materials (YBCO) Variable growth rate Epitaxy at low temperature Atoms arrive in bunches, allowing for much more controlled deposition Greater control of growth (e.g., by varying laser parameters) Disadvantages of PLD • Uneven coverage • High defect or particulate concentration • Not well suited for large-scale film growth • Mechanisms and dependence on parameters not well understood Ways to deposit thin films substrate target substrate Chemical vapor depositionCVD Ar+ target Sputtering Evaporation (Molecular beam epitaxyMBE) substrate gas Low energy deposition (MBE): ~0.1 eV High energy deposition (Sputtering ~ 1 eV) may get islanding unless you pick right substrate or heat substrate to high temperatures smoother films at lower substrate temperatures, but may get intermixing Low energy deposition (MBE): ~0.1 eV High energy deposition (Sputtering ~ 1 eV) may get islanding unless you pick right substrate or heat substrate to high temperatures smoother films at lower substrate temperatures, but may get intermixing Molecular Beam Epitaxy Outline Epitaxy Molecular Beam Epitaxy Molecular Beam Problems and Diagnostics Epitaxy Method of depositing a monocrystalline film. Greek root: epi means “above” and taxis means “ordered”. Grown from: gaseous or liquid precursors. Substrate acts as a seed crystal: film follows that ! Two kinds: Homoepitaxy (same composition) and Heteroepitaxy (different composition). Epitaxy Homoepitaxy: # To grow more purified films than the substrate. # To fabricate layers with different doping levels Heteroepitaxy: # To grow films of materials of which single crystals cannot be grown. # To fabricate integrated crystalline layers of different materials Epitaxy Vapor Phase Epitaxy (VPE) SiCl4(g) + 2H2(g) ↔ Si(s) + 4HCl(g) (at 12000C) # VPE growth rate: proportion of the two source gases Liquid Phase Epitaxy (LPE) Czochralski method (Si, Ge, GaAs) # Growing crystals from melt on solid substrates # Compound semiconductors (ternary and quaternary III-V compounds on GaAs substrates) Molecular Beam Epitaxy (MBE) # Evaporated beam of particles # Very high vacuum (10-8 Pa); condense on the substrate #Beam particles do not interact with each other Molecular Beam Epitaxy Molecular Beam Epitaxy: Idea ! Objective: To deposit single crystal thin films ! Inventors: J.R. Arthur and Alfred Y. Chuo (Bell Labs, 1960) Very/Ultra high vacuum (10-8 Pa) Important aspect: micron/hour) Slow deposition rates require proportionally better vacuum. slow deposition rate (1 Molecular Beam Epitaxy: Process Ultra-pure elements are heated in separate quasi-knudson effusion cells (e.g., Ga and As) until they begin to slowly sublimate. Gaseous elements then condense on the wafer, where they may react with each other (e.g., GaAs). The term “beam” means the evaporated atoms do not interact with each other or with other vacuum chamber gases until they reach the wafer. Molecular Beam A collection of gas molecules moving in the same direction. Simplest way to generate: Effusion cell or Knudsen cell Oven Sample Orifice Test Chamber Pump Knudson cell effusion beam system Molecular beam Oven contains the material to make the beam. Oven is connected to a vacuum system through a hole. The substrate is located with a line-of-sight to the oven aperture. From kinetic theory, the flow through the aperture is simply the molecular impingement rate on the area of the orifice.