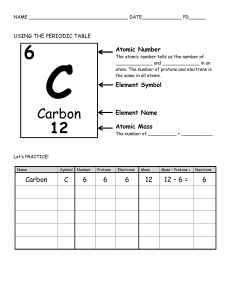
1 SCORE: /40 PHYSICAL SCIENCE: WEEK 1 Instructions: On one whole sheet of paper, complete the activities below. You can work individually, in pairs, or in trios. Don’t forget to write your name and section on your paper. PART 1: The BIG BANG THEORY (20 pts) Rewatch the videos below and in your own words, create a timeline of the creation of the universe. The timeline should contain the following: A. Stages of the Big Bang Theory B. Significant events happened in every moment after the Big Bang. Videos: 1) The Beginning of Everything -- The Big Bang: https://youtu.be/wNDGgL73ihY 2) Origins of the Universe 101 | National Geographic: https://youtu.be/HdPzOWlLrbE Answer the following questions (6 pts): A. Among theories about the origin of the Universe, why does the Big Bang Theory is the most widely accepted? B. Was there really a "bang" in Big Bang? Explain. PART 2: Elements and the Periodic Table (14 pts) Study the text and examples and use your periodic table of elements to complete the table below. ATOMS, ISOTOPES & IONS In an atom, protons and neutrons are the two massive subatomic particles located at the central portion called nucleus. These two particles in an atom create the identity of the atom. The identity of the neutral atom is conventionally represented as shown in the right: The symbol of the elements consists of one or two letters that always starts with a capital letter. The symbol Z is the atomic number which is equal to the number of protons (p+) and determines the amount of positive charge in the nucleus. A corresponds to the mass number, which is equal to the sum of the number of protons (p+) and neutrons (n0). e- are electrons. Below are necessary relationships for calculations: Z = p+ p+ = e- (neutral) p+ > e- (cation) p+ < e- (anion) A = p+ + n 0 p+ = A - n0 The chemical behavior of an atom is determined by the electrons and protons. The position of the electron in the atom subjects it from being lost or gained during chemical interactions. During such events, the neutral atom changes to a charged particle known as ion. The positively charged particle is an atomic +2 32 −2 symbol but a charge is added on the upper right hand corner (e.g. 40 20𝑀𝑔 , 16𝑆 ). The nuclear behavior of the atom is determined by protons and neutrons. These particles define the existence of isotopes. Isotopes are elements with the same atomic number (number of protons but different in atomic mass due to differences in the number of neutrons (e.g. 126𝐶 𝑎𝑛𝑑 146𝐶 ). On the left, is an illustration of the three hydrogen isotopes. Different industries rely on the use of some isotopes such as Co-60 in radiation therapy for cancer, C14 in studies of metabolic changes, Na-24 as pipe leak locator, and U-235 as source of energy in nuclear power plants. Copy and answer the table: Symbol 52 24𝐶𝑟 197 79𝐴𝑢 Name of Element/ Isotope Chromium Gold Z 24 79 20 #p+ 24 79 #n0 28 118 32 16𝑆 List three (3) elements and its functions which are important for the human body: 1) 2) 3) A 52 197 40 #e- 21 78