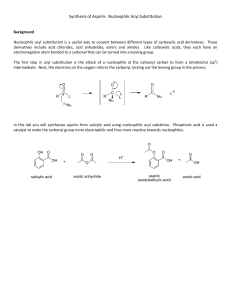
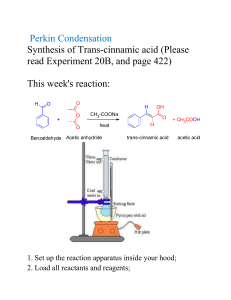
Friedel-craft’s acylation of anisole Introduction: The purpose of this experiment is to perform Friedel-Crafts acylation of anisole, and to determine whether this would yield the para-, ortho-, or meta-isomer of methoxy acetophenone, or a mixture of the isomers. A Friedel-Craft reaction is an example of a substitution of aromatic compounds in which a new carbon-carbon bond is formed by the substitution of an alkyl or an acyl (COCH3) group. In this experiment we will use Aluminum chloride as a catalyst and acetic anhydride as the acetylating agent. The aluminum chloride can act as a Lewis acid by receiving an electron pair from the acetic anhydride. This causes the acetic anhydride to split into an acetate ion and a potential acetyl group with a carbocation (the acylium ion). After this work up step, the acylium ion reacts with the aromatic ring of anisole in a twostep substitution with the formation of methoxy acetophenone. Theoretically there is the possibility that the acetyl group will be added to any carbon on the benzene ring which could result in the formation of the meta, para and ortho, isomers. It is expected that, one product can be predominantly forming due to the activating nature of the substituent present in anisole(-OCH3). The alkoxy group can activates the benzene ring and directs the electrophilic acyl group to the para position due to its electron donor capability. However, the possible products can be identified by the different absorption bands on the IR and 1H NMR spectra due to different amount of adjacent hydrogen atoms to the substituted groups. Reaction Diagram, Mechanism and Structures of Relevant Molecules: Formation of intermediate acylium ion: Methoxy group as para directing. Methoxy group as ortho directing. Reagent Table & Theoretical Yield Calculation: Name Formu la MW (g/m ol) MP (°C) BP (°C) Densi ty (g/ml ) 1.000 at 3.98° C 1.325 at 25°C 1.082 Weight (g) Volu me (ml) mmoles Water H2O 18.02 0 100 Dichloromethane (Methylene Chloride) Acetic Anhydride CH2Cl2 84.93 -97 39.840 13.25 10 156 C4H6O 102.1 -73 140 1.082 1 10.6 AlCl3 133.3 193 2.44 2.9 318 Sublim es 1390 NaOH MgSO 4 40 120.3 7 2.13 2.66 0.6 NaCl HCl 58.44 36.46 801 -30 1413 >100 C7H8O 108.2 -38 155 2.165 1.18 at 25°C 0.996 Anisole 4'Methoxyacetophe none C9H10 O2 150.2 39 258 1.082 3 Aluminum Chloride Sodium Hydroxide Magnesium Sulfate, Anhydrous Sodium Chloride Hydrochloric Acid 22 5 15 5 1.08 1.09 10 1.502 (Theoreti cal yield) 1.39 10 (Theoreti cal yield) Operations Flowchart, Diagrams, & Apparatus Set-up - Step-by-step Experimental Procedure: Reaction: Assemble an apparatus for addition under reflux using a 25-mL round-bottom flask. Fit the condenser with a Y-adapter (provided) to trap HCl gas via an inverted longstem funnel. One end of the Y-adapter should be clasped, while the other end be attached to the acid trap (an inverted long-step funnel fitted over a beaker filled with water). The trap dissolves escaping HCl in water. Connect the Y adapter on the condenser with a straight tube and a thermometer adapter. Place a stir bar in the 25 mL round bottom flask. Assemble the glassware and add the reagents to the flask. Weigh 10.0 mmol anisole and combine it with 10 mL DCM (as a solvent) in a round bottom flask. Collect AlCl3 (2.9 g, 22 mmol) which would be provided in glass vials. Transfer AlCl3 to the round bottom flask. If necessary, use an extra 5 mL solvent, DCM, to rinse residual AlCl3 from the vial into the flask. And add a stir bar or boiling chips, to the reaction flask. Quickly attach the round bottom flask to the condenser and start the stirring Reassemble the apparatus under the hood, measure the Acetic anhydride (1 mL, 11 mmol) into a separatory addition funnel, stopper the funnel immediately. Add the acetic anhydride slowly (dropwise) via the addition funnel. After all the acetic anhydride has been added, turn on the heat to the water bath. Have a beaker of cold water ready to moderate the reaction. The ice-cold water can be transferred to the water bath to subside the vigorous boiling if required. Separation and purification: Slowly pour the reaction mixture over 10 g of ice in a 250 mL beaker. Add 10 mL distilled water to a graduated cylinder and place the cylinder in an ice bath. Use ice-cold water to transfer any residue from the round bottom flask to the beaker. Use a glass stir rod to break-up the ice and fully dissolve the contents of the beaker. Transfer the mixture to a separatory funnel. Drain the aqueous layer from the separatory funnel. Wash the organic layer (DCM) with 5 mL 3M NaOH followed by 5 mL saturated aq. NaCl and remove the aqueous layer after each step. Dry the organic layer over MgSO4 and gravity filter off the contents into a preweighed round bottom flask. Use a rotavap to remove the organic solvent. Weigh the final product and record its mass. If the product remains a liquid at room temperature, we can’t acquire its melting point (mp 38-39 ºC) measurement. Analysis: For TLC analysis, develop a plate of the starting materials (acetic anhydride, anisole), alongside the synthesized final product and co-spot lane. To spot the plate with the product, dissolve a portion of it in dichloromethane (DCM). The developing solvent of 90% hexanes and 10% ethyl acetate and the standard starting materials would be provided by TA. Cheat sheet notes: Clamp the neck of the round-bottom flask and use blue keck clips to secure all joints. Your TA and/or instructor must review the apparatus set-up before you turn on the water to the condenser. Anhydrous aluminum AlCl3 can deactivated by water; protect it from atmospheric moisture and be sure that your glassware is free of residual water and acetone. Avoid contact with AlCl3, acetic anhydride and DCM and do not breath their vapours. Keep acetic anhydride away from water.