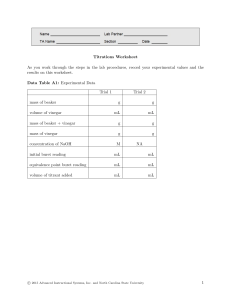
Experiment: Determining the Acetic Acid in Vinegar by Titration For this experiment it is best to choose a "white vinegar" (that is, a colourless vinegar) rather than a "brown vinegar". The colour of a "brown vinegar" can mask the colour change at the end point of the titration. When a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the pH at the equivalence point will be greater than 7. Suitable indicators for this experiment are phenolphthalein or thymol blue. Procedure to titrate the acetic acid in vinegar: 1. Rinse a clean 250 mL conical (erlenmeyer) flask with water. 2. Rinse a clean 25.00 mL pipette (pipet) with vinegar. Pipette 25.00 mL of vinegar into the 250 mL conical (erlenmeyer) flask. 3. Add 2 drops of phenolphthalein indicator to the vinegar. (The solution will remain colourless) 4. Rinse a clean 50.00 mL burette (buret) with standardised 1.00 mol L-1 aqueous sodium hydroxide solution. Fill the burette (buret) with this standardised 1.00 mol L-1 NaOH(aq). 5. Set up the equipment as in the diagram on the right. 6. Run NaOH(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to pink. 7. Repeat the titration carefully several times until concordant titres are achieved. Record you results below then use them to calculate the concentration of acid in the vinegar