Indian Institute of Technology Roorkee
Department of Metallurgical and Materials Engineering
MT-207 Electrical and Electronic Materials
Tutorial 1
1.
i.
Consider a multicomponent alloy containing N elements. If w 1, w2, w3,…..,wN are
the weight fractions of the components 1, 2, 3, …..,N in the alloy and M 1, M2,
M3,……..,MN are the respective atomic masses of the elements, show that the atomic
fraction of the ith component is given by,
ni =
ii.
2.
w i ∕ Mi
-----------------------------w1 ∕M1+w2 ∕M2+------------+wN ∕MN
Consider the semiconducting II-VI compound cadmium selenide, CdSe. Given the
atomic masses of Cd and Se, find the weight fraction of Cd and Se in the compound
and grams of Cd and Se needed to make 100 grams of CdSe.
Explain the general bonding principle of atoms to form a crystalline solid with the
help of energy verses inter-atomic distance plot.
3.
i.
ii.
iii.
iv.
v.
vi.
vii.
4.
Calculate the following:
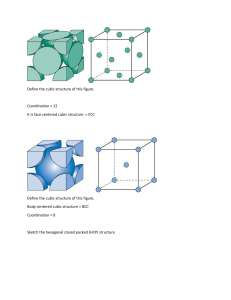
Effective number of atoms in SC, BCC, FCC, HCP unit cells
Relationship between the size of the unit cell and atomic diameter in SC, BCC,
FCC, HCP unit cells
Packing factors of BCC, FCC, HCP unit cells
Packing factor of a diamond cubic crystal structure
Coordination numbers of BCC, FCC, HCP crystal lattice
c/a ratio for an ideal HCP unit cell
Volume of unit cell of germanium in cubic meters, the atomic radius of Ge having
Diamond Cubic structure being 1.223 Ao
Show with the help of neat sketches the following:
i.
Planes whose Miller indices are (111), (210), (010), (0 Ī Ī), (002), (130), (212) and(3
Ī 2).
ii.
Directions whose Miller indices are [111], [110], [1Ī0], [122], [301], [201] and [2 Ī
3].
iii.
[1210], [01 Ī0], [Ī011] directions and (1210), (Ī Ī 22), (1230) planes (Miller Bravais
Index) in HCP unit cell
In a cubic unit cell the (hkl) & [hkl] are perpendicular to each other
5.
6.
7.
8.
iv.
Miller index of the direction that is common to both planes (110) and (111) inside
the unit cell of a cubic crystal.
v.
3 parallel planes of belonging to {111} inside a cubic unit cell (may be touching the
UC).
vi.
6 direction <110> on any one {111}
i.
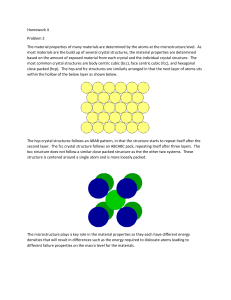
Given the Si lattice parameter a=0.543 nm. Calculate the number of Si atoms per
unit volume, in nm-3.
ii.
Calculate the number of atoms per m2 on the (100), (110), and (111) planes in the
Si crystal as shown in above figure. Which plane has the maximum number of atoms
per unit area?
i.
Why single crystals are used for electronic applications? Explain methods of single
crystal growth.
ii.
What is epitaxial growth? Explain with one example each of growth for; binary,
ternary and quaternary semiconductor compounds, with the help of Eg vs lattice
parameter of the crystal plot.
iii.
What is the significance of ‘distribution coefficient’ in zone refining?
i.
How amorphous semiconductors are prepared? Give an example.
ii.
Explain how the nonstoichiometeric, ZnO crystal with excess Zn at the interstitial
sites contribute free electron for conduction.
Consider 50% Pb- 50% Sn solder alloy:
i.
f13_07_pg196
Sketch the microstructure of the alloy at various stages as it is cooled from the melt.
What is the importance of this alloy in electrical applications?
ii.
At what temperature does the solid melt? What is the significance of this
temperature?
iii.
What is the temperature range over which the alloy is a mixture of melt and solid?
What is the micro structure of the solid ?
iv.
Consider the solder at room temperature following cooling from 183 0C. Assume
that the rate of cooling from 1830C to room temperature is faster than the atomic
diffusion rates needed to change the compositions of the α and β phases in the solid.
Assuming the alloy is 1 kg. Calculate the masses of the following components in the
solid.
a) The primary α ( proeutectic), b) α in the whole alloy, c) α in the eutectic solid
and
d) β in the alloy ( where is the β phase?)
e) For Pb-40Sn, find the degree of freedom at,
i) liquid region, ii) liquidus, iii) two phase mushy region, iv) solidus and v )at
room temperature.