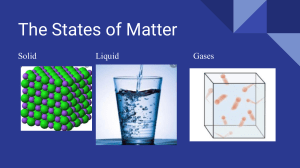
SCIENCE 8 3rd QUARTER Module 1 Does MATTER matter? Most Essential Learning Competencies ⚫ Explain the properties of solids, liquids, and gases based on the particle nature of matter. (S8MT-IIIa-b-8) To the Learner This module was specially designed to help you understand and apply the lesson objectives. Read and follow the simple instructions as your guide. 1. Set a conducive learning space at home so you can focus on your studies. 2. Seek assistance from your parents or guardian to guide you in doing the activities. 3. Take down important concepts and list questions you would like to ask from your teacher. 4. Reflect and apply the concepts that you have learned. The Writer Expectations This module will help you to: • Identify and describe the classification of matter based on its physical state (solids, liquids, and gases). • Differentiate the structure of solids, liquids, and gases based on its mass and volume. • Differentiate the structure of solids, liquids, and gases based on its shape and molecular arrangement (through illustration, etc.). • Differentiate the structure of solids, liquids, and gases based on its movement of molecules (through role-playing, etc.). Pre-test Direction: Read the question carefully and encircle the letter of the correct answer. 1. If an ice cube is left out in an open environment it will change from one state of matter to another. Which is the correct order of the state of matter that the ice cube will go through? a. Solid, Liquid, Gas c. Liquid, Gas, Solid b. Gas, Solid, Liquid d. Solid, Gas, Liquid 2. Which of the following is NOT a physical property of matter? a. Mass c. Volume b. Melting Point d. Flammability 3. Which statement is CORRECT about the molecules of a solid? a. The molecules are randomly moving. b. The molecules are closely packed. c. The molecules are loosely packed. d. The molecules are moving at a fast speed. 4. Marco used a triple beam balance to measure the amount of matter in the stone. He also used a graduated cylinder to measure the space occupied by the stone. What properties of matter are being described in Marco’s activity? a. Mass and Hardness c. Mass and Volume b. Volume and Flexibility d. Volume and Malleability 5. What happens to the molecules of a gas when it is heated? a. It will not move. c. It will move slowly. b. It will slightly move. d. It will randomly move at a fast speed. Looking Back Are You Smarter Than a 7th Grader? Direction: Identify what is being asked by filling the missing letter in the box. 1. It is a pure substance that is made up of one kind of atom. E E N T 2. It is a combination of two or more distinct elements that are chemically combined. C M P U D 2 3. It is the first element in the periodic table. H D O E N 4. It refers to the materials that contain two or more chemical substances dispersed among each other (mixed) and no chemical reactions occur. M X U E 5. It is a homogenous mixture where particles of one substance (the solute) are mixed with the particles of another substance (the solvent). S L T O N 6. It is a type of mixture in which the composition is uniform (one phase) throughout the mixture. H M G E E U S 7. It is a type of mixture in which the composition is not uniform (two or more phases) throughout the mixture. H T R G E E U 8. It refers to the substance that is being dissolved or a substance that exists in a lesser amount. S L T E 9. It refers to the substance in which other material is being dissolved to form a solution and usually exist in greater amount. S O V N 10. It is a type of solution in which a solvent is not capable of dissolving any more solute at a given temperature. S T R T D S L T O N Brief Introduction Matter is anything that occupies space and has mass. It is made up of smallest particles called atom. It was derived from the Greek word “atomos” which means indivisible particle. The study of matter began almost 2,500 years ago where Leucippus and Democritus believed that nature consisted of atoms and the void that surround them. They believed that there are many kinds of atoms and each one has definite sizes and shapes. In 19th century, John Dalton, an English chemist said that all matter was composed of a tiny particle called atoms. An atom is an indivisible building block of matter. Atom is measured in angstrom wherein one angstrom is a unit length equal to one ten-millionth of a millimeter. When atoms are combined in a specific arrangement it is called molecules. Matter has different properties, the chemical and physical property. Chemical property depends on how a substance reacts to another substance like the presence of air, acid, base, water, and other chemicals. The physical property of matter can be classified as intensive and extensive property. The intensive property does not depend on the amount of substance such as color, melting point, boiling point, and density. Extensive property depends on the amount of substance such as mass, volume, length, and shape. The mass is defined as the amount of matter the substance or object has and the measure of space occupied by a substance or object is called volume. 3 Activity 1 Does it Matter? Objective: 1. Identify and describe the classification of matter based on its physical state (solids, liquids, and gases). 2. Differentiate the structure of solids, liquids, and gases based on its mass and volume. Material: Pen Procedure: 1. Identify the physical state of matter of the following samples below by checking the proper box. Sample Salt Granules Table Soft drink Oxygen Carbon Dioxide Cellphone Water Soy Sauce Air Ice Cubes Solid Liquid Gas Guide Questions: 1. Are the samples matter? If not, which sample/s is/are not matter? ________ __________________________________________________________________________ 2. Which samples are solid? ________________________________________________ 3. Which samples are liquid? ________________________________________________ 4. Which samples are gases? ________________________________________________ 5. How do you describe the shape of a solid, does it have a definite shape? How about the liquid and the gas? _____________________________________________ __________________________________________________________________________ 6. Does each sample have a measurable mass? Prove your answer. ___________ ___________________________________________________________________________ ___________________________________________________________________________ 7. Do you think each sample occupies space? Explain your answer. ___________ ___________________________________________________________________________ ___________________________________________________________________________ Activity 2 What MATTER’s Most! Objective: Differentiate the structure of solids, liquids, and gases based on their shape and molecular arrangement (through illustration, etc.). Materials: Pen and Pencil 4 Procedures: 1. Study the illustration below. a. ICE CUBES b. Glass of WATER c.VAPOR from a Hot Coffee Q1. What states of matter are shown in the illustration above? a. ___________________________________________________________________________ b. ___________________________________________________________________________ c. ___________________________________________________________________________ Q2. How do you describe the shape of each sample of matter? Do the ice cubes have a definite shape? How about the water? How about the vapor? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Q3. Suppose you inflate a balloon, do the air particles take now the shape of the balloon? Did the air particles change in shape? ______________________________________________________________________________ Q4. Look at figure A again. What do you think makes a solid to have its shape? Does it have something to do with its particles? How do you describe the particles of a solid? ___________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Q5. Now, looking at figure B, what do you think makes a liquid to have an indefinite shape? Does it have something to do with its particles? How do you describe the particles of a liquid? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Q6. Lastly, look at figure C. What do you think makes a gas to have an indefinite shape? Does it have something to do with its particles? How do you describe the particles of a gas? ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 2. Describe the particles (molecules) of a solid, liquid, and gas using an illustration. Draw your illustration on the assigned object in each state of matter. Solid Liquid Gas 5 Solids are hard because they are composed of tightly packed molecules. Solids have also definite shape and volume. The molecules in a liquid, however, are more fluid. Liquids do not have a definite shape because they only take the shapes of their container but they have definite volume. Gases are composed of high-energy molecules that are constantly moving around because they don’t have a definite shape until they are forced to take the shape of a container. Gases will also take the volume of their container. Activity 3 Moving or Not Moving? Objective: Differentiate the structure of solids, liquids, and gases based on its movement of molecules (through role-playing, etc.). Materials: Pen, Block, Balloon or Plastic, Cup of Water, Plastic Container or Glass Procedures: Set-up 1 1. Examine a single piece of a block. Observe what happens when you roll it on a flat surface. Q1. Did the block change its shape? _________________________________________ Q2. Based on your observation, are the particles of solid moving? If yes, describe how it moves? _______________________________________________________________ ______________________________________________________________________________ Set-up 2 2. Pour a cup of water into a plastic container or a glass. Observe the flow of water. Q3. Did the water take the shape of the container? ______________________________________________________________________________ Q4. Do the particles of water move? If yes, describe how they move? ______________________________________________________________________________ ______________________________________________________________________________ Set-up 3 3. Inflate a balloon or a plastic (ice bag or small plastic). Make sure that the balloon or plastic is not overinflated. Q5. What do you feel as you inflate the balloon or the plastic? ________________ _____________________________________________________________________________ 4. Squeeze the inflated balloon or the plastic. Observe what happens to the balloon or the plastic after squeezing it. Q6. Do the gas particles move? If yes, describe how it moves? ______________________________________________________________________________ ______________________________________________________________________________ 6 Remember • Matter is anything that occupies space and has mass. It is also made up of tiny particles called atom. These particles are moving at all times and have spaces between them. These particles also attract each other. • Characteristics of Solid, Liquid, and Gas A solid has a fixed shape and volume. It is also rigid which means particles are locked into place. It is not easily compressible. It has a little free space between particles. It does not flow easily. A liquid assumes the shape of its container. Liquid particles can move or slide past one another. It’s not easily compressible. There is a little free space between particles. It flows easily. Gases assumes the shape of its container. The particles of gas can move freely. It is easily compressible. There is a lot of free space between particles. Figure 1: Particles of Solid, Liquid, and Gas Check Your Understanding A. Complete the following table to describe the three states of matter. Mass Shape Volume Molecular Arrangement Solid Definite Liquid Gas Indefinite shape Fixed Volume Closely packed Movement of Particles Particles can move/slide past one another B. Explain the following statements. 1. Solids have a definite shape because __________________________________________ ______________________________________________________________________________ 2. Liquids can easily flow because _______________________________________________ 7 ______________________________________________________________________________ 3. Gas can easily be compressed because ________________________________________ ______________________________________________________________________________ Post-test Direction: Read the questions carefully and encircle the letter of the correct answer. 1. If water is placed in a freezer it will change from one state of matter to another. Which of the following shows the correct change of matter that the water will go through? a. Solid to Gas c. Gas to Liquid b. Liquid to Solid d. Solid to Liquid 2. Which of the following is a property of matter? a. Mass and Volume c. Volume and Energy b. Melting Point and Work d. Force and Acceleration 3. Which statement is CORRECT about the molecules of a gas? a. The molecules are randomly moving. b. The molecules are closely packed. c. The molecules are loosely packed. d. The molecules are moving in a low speed. 4. What state of matter is shown in the illustration? a. Gas c. Solid b. Plasma d. Liquid 5. What happens to the particles of water when it is accidentally spilled on the ground? a. It will not flow. c. It will evaporate. b. It will flow easily. d. It will turn into ice. Reflection Direction: Fill up the cards by answering what is being asked to you. Write three (3) things you learned in this module …… ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________ Write two (2) things that still confusing to you …… ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________ Write one (1) thing you enjoyed in the lesson …… _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________ 8 Answer Sheet Name: ________________________________ Grade and Section: ___________________ LOOKING BACK PRETEST 1._________ 2._________ 3._________ 4._________ 5._________ Total Score: ________________ 1.__________________ 2.___________________ 3.___________________ 4.___________________ 5.___________________ 6. __________________ 7. __________________ 8. __________________ 9. __________________ 10. _________________ ACTIVITY 1: Does it matter? Part 1 (Procedure) Sample Salt Granules Table Softdrink Oxygen Carbon Dioxide Cellphone Water Soy Sauce Air Ice Cubes Solid Liquid Gas Part 2 (Guide Questions) 1. 2. 3. 4. 5. 6. 7. ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ACTIVITY 2: WHAT MATTER’S MOST! Part 1 Q1. a. ________________ b. _______________ c. ______________ Q2.______________________________________________________________________________ Q3.______________________________________________________________________________ Q4.______________________________________________________________________________ Q5.______________________________________________________________________________ Q6. _____________________________________________________________________________ Part 2 9 ACTIVITY 3: MOVING OR NOT MOVING! Q1. Q2. Q3. Q4. Q5. Q6. Q7. ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ CHECK YOUR UNDERSTANDING Mass Shape Volume Molecular Arrangement Solid Definite Liquid Gas Indefinite shape Fixed Volume Closely packed Movement of Particles Particles can move/slide past one another 1. Solids have definite shape because ___________________________________________ ______________________________________________________________________________ 2. Liquids can easily flow because _______________________________________________ ______________________________________________________________________________ 3. Gas can easily compress because _____________________________________________ ______________________________________________________________________________ POST-TEST 1._______ 2. _________ 3. __________ 4. ___________ 5. __________ REFLECTION Write three (3) things you learned in this module …… ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________ Write two (2) things that still confusing to you …… ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________________ ______________ Write one (1) thing you enjoyed in the lesson …… _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________________ _____________ 10