Biochemistry Chapter 3: Organic Compounds & Water Properties
advertisement

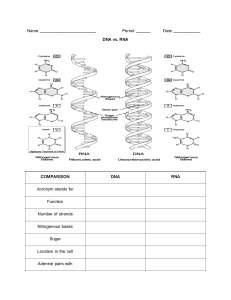
Biochemistry Chapter 3 What does “Organic” mean? One version of “Organic” Organic =Substance having Carbon and Hydrogen Can also have: 1. 2. 3. 4. 5. 6. 7. 8. Phosphorus Sulfur Iron Calcium Sodium Chlorine Magnesium Potassium Organic Compounds • Glucose (C6H12O6) • CH4 • CH3CH2OH Inorganic Compounds • • • • H20 CO2 H2SO4 NaCl Water Facts • What percent of our body weight is water? 65% Can we live without water? Why Not? Chemical Reactions • Need to occur in water (ie. In solution) Chemical Properties of Water • Cohesion Cohesion in Life Water drop shape Surface Tension Cohesion • High Boiling Point • Can absorb much heat before evaporation • Sweating Chemical Properties of Water • Adhesion – Water molecules attracted to substances other than water – Ex. Capillary Action Classes of Organic Compounds • • • • Carbohydrates Lipids Proteins Nucleic Acids Major Elements C, H, O C, H, O C, H, O, N C, H, O, N Carbohydrates • C, H, O • H:O ratio of 2:1 • Monomer= Most Simple Subunit – Monosaccharide (simple sugar) C6H12O6 • The –ose molecules • Energy role in life Monosaccharide Glucose Monosaccharide Monosaccharide Disaccharides =a double sugar =two simple sugars connected Disaccharides Disaccharides Disaccharides Lactose Oligosaccharides • 3-20 monomers • Found in vegetables • Ex. Raffinose and Stachyose Polysaccharides • Many simple sugars connected • Polymer Polysaccharides Starch Polysaccharides Cellulose Polysaccharides Polysaccharides Glycogen (“animal starch”) How do we connect monomers? Dehydration Synthesis Dehydration Synthesis • Glucose= C6H12O6 • Maltose= C12H22O11 How do we break apart polymers? Hydrolysis Water & Enzymes Uses of Carbohydrates? Lipids • C,H,O • H:O ratio much greater than 2:1 – Ex. C17H35COOH (Stearic Acid) • Examples: – Fats ( ex.Triglycerides), – Oils (Olive, canola), – Waxes (Bees, Ear) – Steroids (Testosterone) – Terpenes (Bio Pigments, Rubber) Monomers of Lipids • Glycerol 3 hydro-carbons with 3 hydroxyl groups Monomers of Lipids •Fatty Acid =chain of hydro-carbons with a carboxyl group at the end Lipid Triglyceride(Polymer) Saturated Unsaturated Dehydration Synthesis to get O-bridge Loss of 3 waters Lipid –Phospholipid (Polymer) Saturated vs. Unsaturated Fats Saturated Fats • • • • • • Butter Lard Whole Milk Other Animal Products Rich diet leads to increased cholesterol Rich diet leads to Heart Disease and Arteriosclerosis Hydrogenated fats =add Hydrogen to liquid unsaturated fats to make them solid saturated fats Uses of fats? •Cushioning •Warmth •Secondary energy source •Part of cell membranes (lubrication) Proteins • C,H,O,N • Make up cartilage, bone, muscles • Make up hormones, antibodies and enzymes Amino Acid or Peptide (Monomer) (R-Group) 20 kinds of amino acids Vary in R-Group How do you connect multiple peptides? Dehydration Synthesis Dipeptide Peptide Bond Polypeptide Chain of amino acids Shape and Proteins • Shape determines your job! • Proteins are not functional until they have reached their final shape • Lose of shape=Lose of function Sequence of Shape Formation 1. Primary Structure= Peptides connected by peptide bonds Sequence of Shape Formation 2. Secondary Structure: Alpha-Helix and Beta Pleated sheet structure held by Hydrogen Bonds Hydrogen Bonding Much H-bonding Less H-bonding Protein Structure 3. Tertiary Structure: Globular or Fibrous shape held in place by Hydrogen bonds, Hydrophobic/Hydrophilic Interactions, ionic bonds, disulfide bonds Protein Shape 4. Quaternary Structure=Multiple polypeptide chains connected Multiple bond types hold chains together Heat Shock Proteins:Molecular Chaperons Heat Shock Proteins • 1962 Ferruccio Ritossa • Heated flies and noticed some genes being expressed • HSPs are an ancient survival mechanism • Activated during stress (heat, cancer,pathogens) • Help protein chains fold • Escorts protein chains to their mates • Protects proteins from falling apart in heated situations • Help recycle damaged proteins Enzymes-Great Examples of Proteins! • Biological Catalysts=affect rate of reaction without being consumed or changed by the reaction • Very Specific • The “ase” people (ex. Maltase, Sucrase..) Note: Reactions can also be catalyzed by RNA Enzymes Active Site Models of Enzyme Action Vs. Lock and Key vs. Induced Fit (Both Occur) Both Reversible (On/Off Switch) Enzyme Inhibition • Sarin Nerve Gas – Permanent blocking – Blocks acetylcholinesterase function – Muscles permanently contracted What affects the speed of enzyme action? 1. Temperature Optimal Temp What affects the speed of enzyme action? 2. pH Optimal pH What affects the speed of enzyme action? 3. Substrate Concentration Video Click Here What affects the speed of enzyme action? 4. Coenzymes (organic ex. Vit. B6, B12, NAD, FAD) and Cofactors (Inorganic ex. Mn, Fe, Mg, Zn) Enzymes lower Activation Energy (Energy to break old bonds) Nucleic Acids • C,H,O,N • Genetic Material=blueprint of body passed from parent to child Monomer • Nucleotide Types of Nitrogenous Bases Pyrimidines Purines Polymer- Nucleic Acid • DNA=Deoxyribonucleic Acid – Genetic Material=blueprint of body passed from parent to child • RNA=Ribonucleic Acid – Rough copy of DNA DNA Structure • Double-stranded alpha helix • Has the sugar, deoxyribose • Structure “discovered” by Watson and Crick (Really Rose Franklin) • All DNA of a human cell lined up would span 2 meters – Diameter of cell’s nucleus only 0.000005 meters… DNA Hydrogen Bonds Phosphodiester Linkages Base Pairing Rules • TAGC Genes Segments of DNA that are the codes for certain characteristics Chromosomes =DNA and Histones • If you were to write out your own genome using four letters to represent the four nucleotides, you would write more than 3 billion letters. Using 12 point font, you would fill about a thousand textbooks RNA • Single-stranded • Has the sugar, ribose • Many forms: transfer RNA (t-RNA), ribosomal RNA (r-RNA), messenger RNA (m-RNA) • Lacks Thymine • Has in place Uracil instead of Thymine RNA RNA act as an enzyme? • Yes! • Ribozymes=catalytic RNA • Can help assemble RNA chains or cut RNA or form peptide linkages