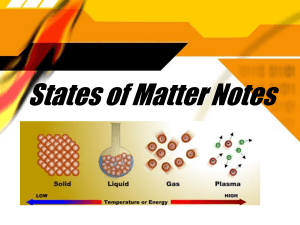
Republic of the Philippines DEPARTMENT OF EDUCATION Division of Rizal MANUEL I. SANTOS MEMORIAL NATIONAL HIGH SCHOOL Taytay, RizaL LESSON PLAN IN SCIENCE 8 Content Standards: The learners demonstrate an understanding of the particle nature of matter as basis for explaining properties, physical changes, and structure of substances and mixtures. Performance Standards: The learners shall be able to present how water behaves in its different states within the water cycle. Learning Competency: Explain the properties of solids, liquids, and gases based on the particle nature of matter. (S8MT - IIIa b - 8) I. LESSON OBJECTIVES At the end of the lesson, the students are expected to: 1. Identify the different phase changes in matter. 2. Determine the various processses that take place in the water cycle. 3. Appreciate the real-life application of physical changes on the phases of matter through the water cycle. II. SUBJECT MATTER Topic: Physical Change III. Learning Resources A. Reference: 1. Teacher’s Guide pages: 2. Learners’ Material pages: Science 8 Quarter 3 Learners Module pages 4-13 3. Textbook pages: 4. Additional Materials: PowerPoint presentation, TV, chalk and blackboard, paper, cold water and laptop. B. Other Learning resources: https://www.slideshare.net/neenaharidas1/physical-chemical-change https://slideshare.net/drmarwa31/physical-and-chemical-changes-of-matter https://youtu.be/CMUmQRgJAo0 https://youtu.be/ncORPosDrjI https://youtu.be/9Pdo0-qkEIY IV. Procedures Teacher’s Activity Daily Routine 1. Prayer Let us all stand, and seek for the guiudance of the Almighty God. I would like to ask Megan to lead the prayer. 2. Greetings “Good afternoon class!” Learner’s Activity Dear God... Amen. Good afternoon ma’am Agpaoa! 3. Securing the cleanliness Before you take your seats kindly pick up the trashes you can see inside the classroom and put it in the trash bins. Arrange your chairs before seating. 4. Checking of attendance “Ms. Secretary May I know who are (The Secretary of the class will stand and tell who are the absentees for the the absentees for this day?” day.) 5. Classroom Norms (Everybody will read) Students will read. A. Review “What have we discussed last meeting?” A. Review previous lesson or presenting a new lesson “Can someone give me a brief information about the properties of solids, liquids and gases according on the particle nature of matter?” “Very good! How about the movement or attraction between their particles?” “Last meeting we discussed about the properties of solids, liquids, and gases based on the particle nature of matter.” “Solids have definite shapes and volume. While, liquids take the shape of their container. Lastly, gases can also take the shape and volume of their container.” “The particles of solid are packed togetehr in fixed position and are held by strong force, therefore the particles cannot move. While in liquid, the particles have spaces between them compared to solid. The particles somehow move as they attract one another. Lastly, in gas, the space between the particles are very large and the attraction is weak which makes the particles move freely.” “Yes, very good! Now that you already understand properties of solid, liquid and gas on the particle nature of matter, let us procedd with our discussion today.” B. Establishing a purpose for the lesson “Before we begin our discussion for today, we will be having a simple activity which will give you a hint about the topic we are going to discuss.” ACTIVITY # 1: HAVE I CHANGED? Materials: 2 pcs of paper Water Sand Transparent bottle/container Instructions: 1. The class will be given three tasks to accomplish within 3 minutes. 2. Upon finishing the task, the students will answer table below using the activity sheet The students will perform the activity. provided by the teacher within 2 minutes. Directions: Put a check ( ∕ ) on the columns below if the materials on the performed task undergone a specific change. Task Rippe d Paper Cramp led paper Mixed with sand and water Change that occur Size Shape Color Question: Are the structure of the materials still the same even if they changed appearance? Are the arrangements of their atoms still the same? Is paper still a paper? C. Presenting examples/ instance of new lesson Student 1: Yes, the structure remains the same. Student 2: Yes, the arrangement of the atoms are still the sme. Student 3: Yes, it is still a paper even though it changes in size and shape. “We have identified from the activity that the materials that were ripped and mixed changed their physical property but the arrangement of their atoms are still the same.” “What do you think is our lesson “Physical changes in terms of today?” arrangement and motion of atoms and molecules.” “Very good!” “ For us to easily understand our lesson, there are terms we need to unlock first. We might encounter these words later during our discussion.” “There are two columns of words flashed on the screen, and you are going to match the terms with the “Yes ma’am!” statements that best describes them. Are my instructions clear?” “Are you ready?” *Will use a powerpoint presentation. “Yes we are.” Condensation – change from gas to liquid Deposition – change from gas to solid Evaporation – change from liquid to gas Freezing – change from liquid to solid Kinetic energy – energy an object has due to its motion Melting – change from solid to liquid Phase change – is the physical process in which a substance goes from one phase to another. Physical change – is a change in appearance of matter without change in its structure. “Now that you have already understand the terms, let us proceed to our activity. This will help us further to understand Physical Changes.” D. Discussing new concepts and practicing new skills #1 “To deepen our understanding about physicla change, let us have another activity.” ACTIVITY # 2:PHYSICAL CHANGE: PHASE CHANGE General Instructions: 1. The students will be given two tasks to accomplish within 8 minutes and upon finishing the tasks, they are going to answer the questions provided in the table below which can also be found on the answer sheet provided by the teacher. TASK 1 Materials: Cold water Transparent Glass Instructions: Pour cold water in a transparent glass until it’s full. Observe the appearance of the glass before and after it is filled with water. TASK 2 Materials: Rubbing alcohol Instructions: Put a small amount of alcohol on you hand and apply it on your arms (skin). Observe what will happen. Repeat if necessary. 2. You will be assessed based on the following criteria: RUBRIC Correctness/Aptness – 50% Timeliness - 30% Active Participation - 25% Total - 100% “Any questions on the rubric?” “None ma’am.” 3. You only have a total of 10 minutes to finish the activity (8 minutes to do the tasks and 2 minutes to briefly answer the questions). “The students who will finish first will receive a price later.” “If there are no more questions with the activity, you may now start.” “Time is up! Let us now check your (Elicit answers from students) Task 1 answers on the guide questions.” Student 1: The surface of the glass is dry before filling it with cold water. Student 2: The surface of the glass became wet after filling it with cold water. Student 3: The cold water caused the surface to become wet due its cold “Very good!” temperature. Task 2 Student 1: The alcohol easily disappeared after being applied to the skin. As it disappears, there is a “Very good!” cooling sensation. Student 2: Yes, it disappears. Student 3: The alcohol disappeared because it evaporated due to change in temperature. Physical change is a change in the form of substance, but does not change it to another substance. It is a change in the appearance of matter without changing its structure. “How can you relate this definition from (Students will share their insights.) our two previous activities?” “Very good! They all undergone physical change.” The ripped paper, crampled paper and mixed sand and water all undergone physicla change becuase even if there is a change on their appearance, the arrangement of their particles is still the same. The paper is still a paper (a solid) after being ripped or crampled and the sand (a solid) and water (a liquid) still has the same arrangements of particles after being mixed. “Do you have questions so far?” “None ma’am.” Moreover, for the cold water and alcohol, there has been a change on their phase. “Again, what are the phases of matter?” “The different phases of matter are solid, liquid, and gas.” “Very good!” “Now let us discuss phase change.” Phase change is a physical process in which a substance goes from one phase to another. “What have you observed before and after you pour cold water on the outer surface of a transparent glass?” “Before pouring cold water on the glass, the outer surface was dry. After pouring cold water, the outer surface became wet and there are some smoke-like appearance on it.” “Very good observation!” “Why do you think this happened? Do you have any idea?” “You are correct. There was a phase hange that occured.” “How about the alcohol that you put on your hands? What were your observations? Why did you feel as it “ It is because a phase change occurs.” Student 1: There is also a phase change that took place. Student 2: As it easily disappears, I felt disappears?” a cooling sensation on my skin “That is a very good observation as well.” “Now, let us thoroughly discuss the phase changes under physical change.” The students will be given two situations. 1. You have two containers filled with water, (a) one with cover and (b) one withput cover. Both were left from 9:00 o’clock in the morning on a hot day until the next 24 hours. Yo noticed that the water in both container decreased, but container b has less water than container a. (Students will share their insights.) Questions: E. Discussion new concepts (new skill#2) 1. What do you think are the changes that took place when the water is left in an open container? In a closed container? (Solicit answer from the students.) 2. What is the particle used on the situation given? “It is water, ma’am.” “Very good, thank you.” “As we all know, water is the most abundant susbstance we can see on our daily life basis. It is everywhere, may it be in sea, ocean, and othert bodies of water. We use it as well to hydrate our body and survive.” “In particle nature of matter, it is said that the particles are always in motion. Water molecules have kinetic energy taht differ from one another. Some particles have higher and some are lower which means some move faster than the other.” “Take note also that it was previoulsy mentioned that the particles of liquid attracts each other, but since the molecules of water differ in kinetic energy, some break away from this attraction and escape from the surface of the liquid. Basically, the molecules that escaped are the ones with higher kinetic energy. The molecules that escape from the liquid and go into gaseous phase is called vapor and in this case, a water vapor. The process by which the molecules on the surface of a liquid break away and change into gas is called evaporation. Do you have any questions so far?” “None ma’am.” “The process where gas changes into liquid is called condensation. It is the reverse of evaporation. This activity is shown in container a wherein the water vapor did not escape because the container is closed. The molecules of the water vapor collided with the cover and accumulate forming droplets. An example of this is the water that collects on the outside of your glass of cold drinks.” “Let us now look at the second situation: 2. You put water in an ice cube tray and left it within the freezer overnight. Upon checking in the morning, you noticed that the water become solid ice. Question: What change do you think occured? (Solicit answer from students.) When you put liquid water inside a freezer, the cooling system of the refrigerator removes heat energy from the water molecules. As a result, they lose and have less kinetic energy and move slowly. Thus, liquid becomes solid.This process is called freezing. Similarly, ice that melts is still water and the process of converting solid into liquid is called melting. “Do you have any questions?” “None so far.” “What are the four phases that we “The four phases are evaporation, condensation, have identified so far?” melting.” “Very good! Let us briefly discussed the two other changes left.” “Have you seen a moth ball?” “What have you observed as you put them in your drawers or closet?” “Yes ma’am.” “The moth balls disappear thorugh time.” “That is correct! This happens because it absorbs heat energy which speeds up the molecules and let them move further. The solid then directly becomes gas, this process is called sublimation. “Do you understand?” “Nice. How about snow? Do you know what snow is?” “Does it occur in oue country?” “Yes it does not occur in our country because we live in a tropical region and the weather is hot. But in some countries snow is formed. And what do you think is the process involved in the formation of snow?” “Yes ma’am.” “Yes ma’am.” “No ma’am.” “Deposition ma’am.” “Great, it is deposition. This process converts vapor (gas) into ice crystals (solid). This happens when the molecules of vapor in air slow down and move closer together, releasing energy.” “Are you all aware of the water cycle?” “Can someone share their idea regarding water cycle?” “Yes ma’am.” “Water cycle is the movement of water within the Eath and atmosphere. It involves different comoplex processes.” “Very good!. That is correct. In fact, some of these complex processes include the phase changes of matter we discussed. Again, what are the six phases of change in matter?” “Thank you very much! That is correct.” “ These are evaporation, condensation, freezing, melting, sublimation and deposition.” F. Developing mastery Divide the students into four groups. Let one of the students pick a paper where the task is written. Tasks are as follows: 1. Make an illustration of water cycle showing the different phase changes. 2. Role-play the behavior of particles in the different phase changes. 3. Create a mnemonics for PHASE CHANGE. 4. Video analysis about physical changes of matter. Link: https://youtu.be/CMUmQRgJAo0 and https://youtu.be/ncORPosDrjI SCORING RUBRICS G. Application Activity Head, Heart, Hands and Feet (3H & F) This activity will test what you learned and understand in this lesson. Complete the chart below by answering the questions. H. Generalization Directions: Fill in the blanks with the correct answer. Write your answers on a separate sheet of paper. Physical change refers to the change in __________ of matter _________ a change in its structure. Phase change is a __________ process in which a substance goes from one ________ to another. There are _________ types of phase change, namely, ___________, ________,___________, __________, _________, and _____________. I. Assessment Directions: On a ½ sheet of paper, kindly answer the following: Physical change refers to the change in appearance of matter without a change in its structure. Phase change is a physical process in which a substance goes from one phase to another. There are six types of phase change, namely, evaporation, condensation, freezing, melting, sublimation, and deposition. G Part A. Identification Identify each face change being shown on the illustration below. Write your answers on the space provided. Part B. Essay Among the 6 phase changes, choose only 3 and briefly expalin in 2-3 sentences how does it occur in the water cycle. The explanation must be based on the particle nature of matter. (3 points each) J. Additional Activities Assignment 1. Define protons, electrons, and neutrons. 2. List 10 atomic elements with their corresponding number of proton, electron, and neutron. 3. Identify the proton, electron and neutron through an illustration and label them. 4. Make an advance reading about the structure of an atom. IV. Remarks V. Reflection A. No. Of learners who got 80%: 5– 4– 3– 2– 1– 0– Total B. No. Of learners who got below 80% (additional activities): C. Did the remedial activity work?: D. No. Of learners to continue remedial activity: E. Teaching strategies that work: F. Difficulties encountered: G. Innovation to share Prepared by: CIRILLE M. AGPAOA Science 8 Teacher Checked by: MARINA R. DE VERA Chairman, Science Department