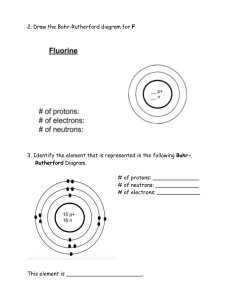
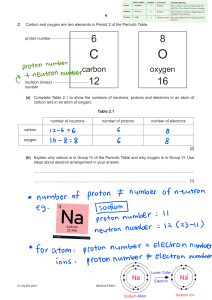
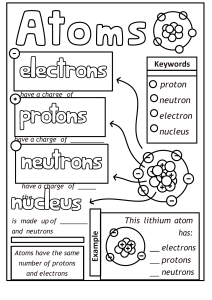
8th Name: Grade Chemistry Unit Review Teacher: Date: Each student should be able to: 8.5(A) Describe the structure of atoms, including the masses, electrical charges, and locations, of protons and neutrons in the nucleus and electrons in the electron cloud. 8.5(B) Identify that protons determine an element's identity and valence electrons determine its chemical properties, including reactivity. 8.5(C) Interpret the arrangement of the Periodic Table, including groups and periods, to explain how properties are used to classify elements. 8.5(D) Recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas containing subscripts. 8.5(E) Investigate how evidence of chemical reactions indicate that new substances with different properties are formed. 8.5(F) Recognize whether a chemical equation containing coefficients is balanced or not and how that relates to the law of conservation of mass. The diagram below is a Bohr Model of a neutral atom. A. Label the diagram with the following words: electron, neutron, nucleus, proton B. Label the charges on the protons, neutrons and electrons. 5. Identify the name of the element above. The atom above is _____________________________. 6. Which element can be identified as having 3 protons?__________________________________. 7. This is the correct model for which element? ______________________. 8. Correctly sequence the following from smallest to largest in mass: protons, atom, electrons. ______________________ _______________________ ________________________ Smallest Largest 9. Which element has 18 protons, 22 neutrons and 18 electrons? 10. Circle which piece of lab equipment below does not belong with the group. Write a complete sentence explaining why it does not go with the group. Graduated cylinder first aid kit fire extinguisher eyewash 11. Complete the table below summarizing the characteristics of subatomic particles. Subatomic Particle Charge Location electron Mass Less than one amu Nucleus + 1 amu 12. A. A valance electron is defined as… B. A valance electron is important because… C. How many valance electrons do each of these elements have? _____ valance electrons _____ valance electrons _____ valance electrons 13. A. On the Periodic Table of Elements, columns are called ______________________; all of these have the same number of ___________________________. elements B. On the Periodic Table of Elements, rows are called _____________________; all of these have the same number of __________________________. C. On the Periodic Table below, the group including helium, neon, argon, krypton, xenon and radon is circled. Label “A”. with the name of this group. Explain why these elements do not react with other elements. D. Shade the nonmetals blue on the periodic table above. What are the physical properties of nonmetals? E. Shade the metals yellow on the periodic table above. What are the physical properties of metals? F. Shade the metalloids red on the periodic table abo ve. What are the physical properties of metalloids? 14. Use your Periodic Table to complete the chart below. 15. A. Which element in in group 4, period 2?____________________ B. Which element is in group 2, period 5?____________________ C. Which element is in group 18, period 3? __________________ 16. You have observed several chemical changes in class over the last few weeks. List evidence of a chemical change (list 4). 17. List 4 examples of physical changes. 18. List 4 “real world” examples of chemical changes. 19. A. The formula for density is ___________________________________. B. The tool needed to measure mass ____________________________. C. The tool needed to measure volume of an irregularly shaped solid __________________. 20. A student finds a mineral and determines the 3 gram nimeral has a volume of 1.15 cm3. What type of mineral did the student find? Show your work. 21. Complete the table below with your knowledge of chemical formulas. 22. A. Write the Law of Conservation of Mass. B. What does it mean for a chemical equation to be balanced? A. B. Chemical Equation Visual Model 23. A. Label the products and the reactants in the chemical equation above. B. Fill out the table using the equation above: Elements found in Equation Na Reactant Side (Number of Atoms) 1 Product Side (Number of Atoms 1 C. Is the equation above balanced (circle 1)? Yes/No 24. Chemical Equation C + O2 CO2 2 Zn + 2 HCl ZnCl2 + H2 6C + 6H2O2 C6H12O6 2HCl + Ca(OH)2 CaCl2 + 2H2O Is it balanced? Yes/No Yes/No Yes/No Yes/No