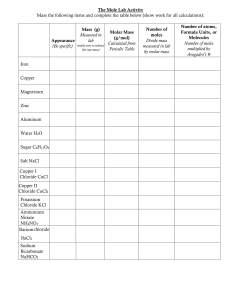
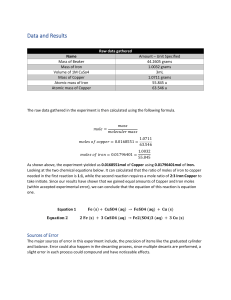
Name ____________________________________________________Hour________2.7.18 THE SINGLE REPLACEMENT LAB OF IRON REPLACING COPPER IN A COPPER (II) CHLORIDE SOLUTION DAY I Read thru procedure and create a data table for the data that needs to be recorded 1. WITH A PERMANENT MARKER WRITE THE NAMES AND HOUR OF THE PEOPLE IN YOUR GROUP ON A CLEAN BABY FOOD JAR 2. MASS THE BABY FOOD JAR ON THE ANALYTICAL BALANCE NEXT DOOR AND RECORD THE MASS 3. ADD around 7.50 GRAMS OF COPPER (II) CHLORIDE TO THE BABY FOOD JAR AND RECORD ACTUAL MASS OF COPPER (II) CHLORIDE 4. ADD 50.0 mL OF WATER TO THE BABY FOOD JAR 5. WITH A SPOON OR STIRRING ROD, STIR THE SOLUTION UNTIL ALL THE COPPER (II) CHLORIDE DISSOLVES RECORD INITIAL OBSERVATIONS 6. TAKE A PIECE OF STEEL WOOL AND POLISH AN IRON NAIL. NO RUST!!!! 7. MASS THE NAIL ON THE ANALYTICAL BALANCE NEXT DOOR AND RECORD THE MASS 8. PLACE THE POLISHED NAIL (HEAD FIRST) INTO THE BABY FOOD JAR CONTAINING THE SOLUTION OF COPPER II CHLORIDE LET THIS SIT FOR TWO DAYS Create an observation table and record observations for day 1 DAY 2 1. WITH FORCEPS, PULL OUT THE REMAINDER OF THE IRON NAIL AND WASH OFF ANY COPPER ON THE NAIL USING A WASH BOTTLE. ALL COPPER MADE NEEDS TO BE IN THE BABY FOOD JAR!!!! 2. PLACE NAILS ON A LABELED PAPER TOWEL WITH YOUR NAMES AND HOUR 3. SIPHON OFF THE SOLUTION OF IRON II CHLORIDE AND PUT IN THE SINK Name ____________________________________________________Hour________2.7.18 BE CAREFUL NOT TO LOOSE ANY COPPER 4. ADD 25 mL OF HCL TO THE BABY FOOD JAR TO WASH THE COPPER 5. SIPHON OFF THE ACID AND PUT INTO THE SINK 6. WASH THE COPPER WITH 25.0 mL DISTILLED WATER THEN SIPHON OFF THE WATER 7. PLACE BABY FOOD JAR WITH THE COPPER IN A DRYING OVEN 8. DRY FOR ONE OR TWO DAYS DAY 3 1. WEIGH THE NAIL AND RECORD THE MASS 2. WEIGH THE BABY FOOD JAR WITH THE COPPER AND RECORD THE MASS SCRAP THE COPPER OUT OF THE JAR WITH A SPATULA AND PLACE INTO A BAGGIE OR THE GARBAGE CAN BABY FOOD JAR NEEDS TO BE GIVEN BACK TO MRS. MALKMUS DO CALCULATIONS! WRITE A BALANCED CHEMICAL EQUATION 1. CALCULATE THE NUMBER OF GRAMS OF IRON NAIL USED 2. CALCULATE THE NUMBER OF MOLES OF IRON USED 3. CONVERT THE MOLES OF IRON TO MOLES OF COPPER USING COEFFICIENTS Name ____________________________________________________Hour________2.7.18 4. CONVERT MOLES OF COPPER TO GRAMS OF COPPER. THIS IS CALLED YOUR THEORETICAL YIELD. ( HOW MUCH YOU SHOULD HAVE MADE) 5. USING YOUR DATA: CALCULATE THE NUMBER OF GRAMS OF COPPER PRODUCED. THIS IS CALLED YOUR EXPERIMENTAL YIELD 6. % YIELD= EXPERIMENTAL YIELD OF COPPER/ THEORETICAL YIELD OF COPPER